CHEMICAL COMPOSITION AND BIOLOGICAL ACTIVITIES OF THE ESSENTIAL OILS AND THE METHANOLIC EXTRACTS OF BUNIUM INCRASSATUM AND BUNIUM ALPINUM FROM ALGERIA
- Bunium,
- Anti-oxidant activity,
- Anti-inflammatory activity,
- Anti-hemolytic activity,
- Antibacterial activity
- Essential oil ...More
Copyright (c) 2017 El Kolli Hayet, Laouer Hocine, El Kolli Meriem

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
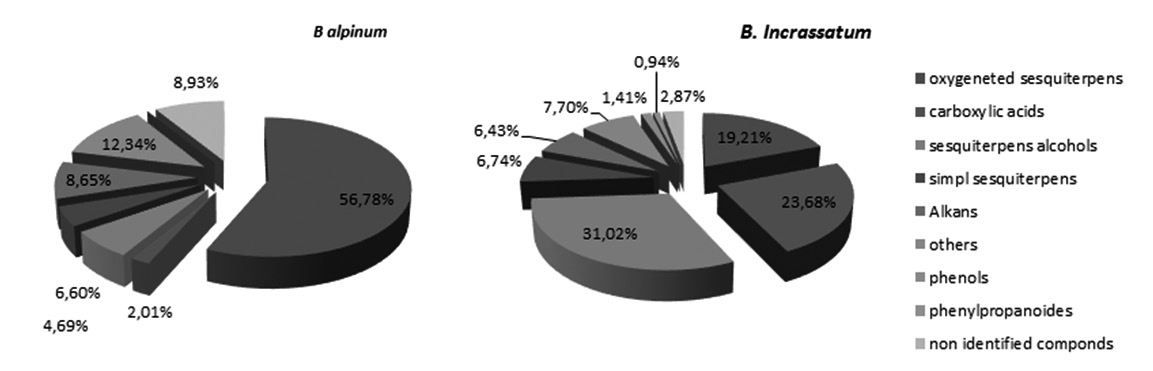
In order to study the curing proprieties of endemic Algerian plants, it is evaluated the chemical composition and four biological activities of two Apiaceae species, which are Bunium incrassatum and Bunium alpinum. The essential oils (EO) obtained by hydro-distillation of dried aerial parts were analyzed by GC/ MS. The antibacterial activity was investigated using the disc diffusion assay against ten (10) Gram-positive and Gram-negative bacteria. Antioxidant activity was evaluated by DPPH technique. Methanolic extracts (Me EXTs) were used in studying in vitro anti-inflammatory activity using egg albumin technique and in vitro anti-hemolytic activity using HBRC technique. The EO yield based on dried plant material was 0.09 % for B. incrassatum and 0.10 % for B. alpinum. Thirty-one compounds (corresponding to 97.19%) were identified for B. incrassatum. The main component was palmitic acid (18.39%). While twenty-four compounds (coresponding 87.33%) were identified for B. alpinum. The main component was caryophyllene oxide (33.84%). The study of antibacterial activity demonstrated that the EOs showed a modest antibacterial activity compared to gentamicin. The antioxidant activity revealed that EOs and Me EXTs demonstrated a very important anti-radical activity compared to standards (BHT, BHA, tocopherol, quercitin and rutin). The Me EXTs demonstrated also, significant antihemolytic and anti-inflammatory activities to those of standard (sodium diclofenac). It was found that all these activities are much related to the chemical composition of EO and Met EXTs. These activities could be exploited in the food industry for food preservation or in pharmaceutical industry.
References
- S. Burt. Int J Food Microbiol 94, 223, (2004).
- G.V .Degtjareva, E.V. kljuykov, T.H. Samigullin, C.M. Valiejo-Roman, M.G. Pimenov. J Linn Soc Bot 160,149, (2009).
- J.V. Shner, T.V. Alexeeva, M.G. Pimenov, W. B.E. Van. S Afr J Bot 626, 6, (2010).
- A. Bousetla, M. Kurkcuoglu, B. Konuklugil, K.H.C. Baser, S. Rhouati. Chem Nat 50, 753, (2014).
- G. Fenu, E. Mattana, A. Congiu, G .Bacchetta. Candollea 65 (2), 347, (2010).
- U.R. Rehman Jamil, M .Ahmad, M.A. Saeed, M. Younas, J. Chem Soc Pak 13 (1), 57, (1991).
- M. Meshkatalsadat, R. Badri, S. Zarei, Int J Pharm Tech Res 1 (2), 129, (2009).
- M. Azimzadeh, R. Amiri, M. H. Assareh, M.R. Bihamta, M. Forootan. J Med Plants Res 6 (7), 1119, (2012).
- R.K. Thappa, S .Gosh, S.G. Agarwal. Food Chem 41, 129, (1991).
- F. F. Charifari, N. Yassa, V. Mozaffarian. Pak J Pharm Sci 23(3), 300, (2010).
- G. Appendino, O.H. Cetin, P .Lusso, M .Ciseros. Phytochem 30 (10), 3467, (1991).
- U. Cakilcioglua, S. Khatunb, I .Turkogluc, S. Hayta. J Ethnopharmacol 137, 469, (2011).
- Leonard A, Lee T and Clyde L. 69 annual meeting of the association of official agricultural chemists. Oct 10-12. Washington (1955).
- H. Laouer, M. El Kolli, S, Prado, N. Baldovini. Phytother Res 23 (12), 1726, (2009).
- S. Athamena, I. Chalghem, A. Kassah-Laouar, S. Laroui, S. Khebri, Lebanese Science Journal 11,1, (2010).
- H. Li, K.W. Cheng, C.C .Wong, K.W. Fan, F. Chen, Y. Jiang. Food Chem 102, 771, (2007).
- A.W. Bauer, W.M. Kirby, J.C. Sherris, M.Turck. Am J Clin Pathol 45, 493, (1966).
- G .Singh, P. Marimuthu, C.S. De Heluani, C. A. N. Catalan. J. Agric. Food Chem 54,174, (2006).
- M. Barkat, I. Laib. Revue de génie industriel. 6, 46, (2011).
- P. Padmanabhan, S. N. Jangle. Int J app Basic Med Sci 2(1), 109, (2012).
- F. Alhakmani, S. Kumar, S. A. Khan. Asian Pac J Trop Biomed 3(8), 623, (2013).
- W. M. Nishanthi, M. Vijey Aanandhi, K. Azhagesh Raj, B. Vijayakumar. Journal of Pharmacological Screening Methods 2, 88, (2012).
- O. T. Kolawole, M. O. Akiibinu, A. A. Ayankunle, E. O. Awe. Br J Med Med Res 3 (2), 216, (2013).
- R. Omidbaigi, A. J. Arvin. J Essent Oil Bear Pl 12, 34, (2009).
- B. H. Naghdi, D. Yazdani, S. Mohammad Ali, F. Nazari. Ind Crops Prod 19, 231, (2004).
- F. Sandberg, D. Corrigan. Natural Remedies; Their Origins and Uses. Taylor & Francis e-Library. (2004).
- A. Rahman, M.I. Choudhary, S. Hayat, A.M. Khan, A. Ahmad, S. Malik. Phytochem. 52, 495, (1999).
- F. Bakkali, S. Averbeck, D. Averbeck, M. Idaomar. Food Chem Toxicol .46, 446, (2008).
- S. Khosravinia, S. M.Ziaratnia, A. Bagheri, G Rajabzadeh, S. H. Marashi. Not Sci Biol. 4, 49, (2012).
- C.C. Seema, S.V. Sharan, R.B. Srinivasa, V. Meena. Rasayan J Chem. 4, 457, (2011).
- M.M. Tajkarimi, S.A. Ibrahim, D.O. Cliver. J Sci Food Agr 76(2), 270, (2010).
- S.G.Griffin, S.G. Wyllie, J.L. Markham, D.N. Leach. Flavour Fragr J. 14, 322, (1999).
- H. Baydar, O. Sagdic Ozkan, T. Gand Karadogan. Food Control. 15, 169, (2004).
- M. El kolli, H. Laouer, H. El Kolli, S. Akkal, F. Sahli. Asian Pac J Trop Biomed. 6(1), 8, (2016).
- B. Tepe, D. Daferera, A. Sokmen, M. Sokmen, M. Polissiou. Food Chem. 90, 333, (2005).
- N. Benhammou, F. A. Bekkara, T. K. Panovska. C R Chimie. 12,59, (2009).
- F. Lu, L.Y. Foo. Food Chem.75, 197, (2001).
- T. Nalina, Z. Rahim. Chem Biochem. 3, 10, (2007).
- Bruneton J. Pharmacognosie et Phytochimie des Plantes Médicinales. Tec & Doc. Paris. (1999).
- Multon J. L. Additifs et Auxiliaires de Fabrication dans les Industries Agroalimentaires, Lavoisier, Paris. (2002).
- T. Kulisic, A. Radonic, V. Katalinic, M. Milos. Food Chem. 85, 633, (2004).
- S .Chandra, P .Chatterjee, P. Dey, S. Bhattacharya. Asian Pac J Trop Biomed. S178, (2012).
- S .Sachin, S. Archana, R. Juvekar, M. N. Gambhire. Int J Pharm Pharm Sci 2 (1),146, (2010).
- J. Pastre, N. Priymenko N. Revue Med Vet 158 (4), 180, (2007).
- J. Neuzil, R. Stocker. J Biol Chem 269 (24), 16712, (1994).
- D. W. Appleton, B. Sarkar B. J Biol Chem 246 (16), 5040, (1971).
- M. Bourkhis, M .Hnach, J. Paolin, J. Costa, A. Farah, B. Satrani. Bulletin de la Société Royale des Sciences de Liège 79, 141, (2010).
- D. Chakraborty, B. Shah. Int J Pharm Pharm Sci 3 (3), 192, (2011).
- F. Dai, Q. Miao, B. Zhou, L. Yang, Z.L. Liu. Life Sci 78, 2488, (2006).
- M. Blasa, M. Candiracci, A. Accorsi, M. P. Piacentini, E. Piatti. Food Chem 104 (4), 1635, (2007).
- J. Tabart, C. Kevers, J. Pincemail, J. O. Defraigne, J. Dommesa. Food Chem 113, 1226, (2009).


