- Perovskite,
- Nanocatalyst,
- Sec-amines,
- Reduction
Copyright (c) 2017 Hossein Bavandi, Ali Shiri, Haman Tavakkoli

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
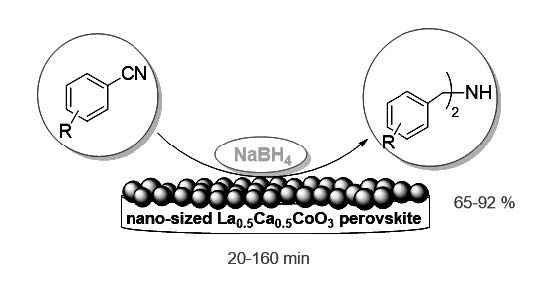
Nano-sized La0.5Ca0.5CoO3 perovskite, which was produced via the sol-gel method, was an efficient heterogeneous catalyst in combination with NaBH4 for the rapid chemoselective reduction of aryl nitriles to bis-(benzyl)amines at 40 ºC in good to excellent yields. The physico-chemical properties of the catalyst were characterized by means of differential thermal analysis (DTA), thermogravimetric analysis (TGA), X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDX) and particle size distributions images. The results show that nanoparticles have regular shapes with well-defined crystal faces with an average size of 30 nm.
References
- F. Ullman, Ullmann’s Encyclopedia of Industrial Chemistry, Wiley- VCH, Weinheim, Germany, 2008.
- S.A. Lawrence, Amines: Synthesis, Properties and Application, Cambridge University Press, Cambridge, U.K., 2004.
- S.S. Insaf , D.T. Witiak, Synthesis, 3, 435, (1999).
- Z. Rappoport, The Chemistry of the Cyano Group, Wiley Interscience, New York, 1970.
- J. March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure Wiley Interscience, Toronto, Canada, 1992.
- D. Addis, S. Enthaler, E. K. Jung, B. Wendt, M. Beller, Tetrahedron Lett. 50, 3656 (2009).
- S. Enthaler, D. Addis, K. Junge, G. Erre, M. Beller, Chem. Eur. J. 14, 9491 (2008).
- E.R.H. Walker, Chem. Soc. Rev. 5, 23, (1976).
- B. Klenke, I.H. Gilbert, J. Org. Chem. 66, 2480, (2001).
- C.A. Buehler, D.E. Pearson, Survey of Organic Synthesis, Wiley- Interscience, New York, 1970, p. 413.
- O. Mitsunobu, Comprehensive Organic Synthesis; Trost, B. M. Fleming, I., Eds.; Pergamon: Oxford, U.K., 1991; Vol. 6, p 65.
- H.C. Brown, J.S. Cha, J. Org. Chem. 58, 3974, (1993).
- M. Hudlicky, Reductions in Organic Chemistry, Second ed., ACS Monograph 188, American Chemical Society, Washington, D.C, 1996, p. 19.
- N.M. Yoon, H.C. Brown, J. Am. Chem. Soc. 90, 2927, (1968).
- R.C. Wade, J. Mol. Catal. 18, 273, (1983).
- J.M. Khurana, G. Kukreja, Synth. Commun. 32, 1265, (2002).
- B. Ganem, J.O. Osby, Chem. Rev. 86, 763, (1986).
- H.C. Brown, Boranes in Organic Chemistry, Cornell University Press, New York, 1972, pp. 209-251.
- C.D. Chandler, C. Roger, M.J. Hampden-Smith, Chem. Rev. 93, 1205, (1993).
- R. Robert, M.H. Aguirre, P. Hug, A. Reller, A. Weidenkaff, Acta Mater. 55, 4965, (2007).
- M. Nandia, K. Sarkara, M. Seikhc, A. Bhaumik, Microporous Mesoporous Mater. 143, 392, (2011).
- H. Aono, E. Traversa, M. Sakamoto, Y. Sadaoka, Sens. Actuators, B: Chem., 94, 132, (2003).
- R. Horyn, R. Klimkiewicz, Appl. Catal., A: Gen., 370, 72, (2009).
- N. Pal, M. Paul, A. Bhaumik, Appl. Catal., A: Gen., 393, 153, (2011).
- M. Yazdanbakhsh, H. Tavakkoli, S. M. Hosseini, Desalination., 281, 388, (2011).
- H. Tavakkoli, M. Yazdanbakhsh, Microporous Mesoporous Mater., 176, 86, (2013).
- A.V. Salker, N.J. Choi, J.H. Kwak, B.S. Joo, D.D. Lee, Sens. Actuators, B: Chem. 106, 461, (2005).
- M.D. Smith, A.F. Stepan, C. Ramarao, P.E. Brennan, S.V. Ley, Chem. Commun. 21, 2652, (2003).
- S.P. Andrews, A.F. Stepan, H. Tanaka, S.V. Ley, M.D. Smith, Adv. Synth. Catal. 347, 647, (2005).
- S. Lohmann, S.P. Andrews, B.J. Burke, M.D. Smith, J.P. Attfield, H. Tanaka, K. Kaneko, S.V. Ley, Synlett, 8, 1291, (2005).
- G. Pilania, P.X. Gao, R. Ramprasad, J. Phys. Chem. 116, 26349, (2012).
- M. Yazdanbakhsh, I. Khosravi, M.S. Mashhoori, M. Rahimizadeh, A. Shiri, M. Bakavoli, Mater. Res. Bull. 47, 413, (2012).
- T. Sanaeishoar, H. Tavakkoli, F. Mohave, Appl. Catal. A, 470, 56, (2014).
- A. Shiri, F. Soleymanpour, H. Eshghi, I. Khosravi, Chin. J. Catal. 36, 1191, (2015).
- C.F. Kao, C.L. Jeng, Ceram. Int. 25, 375, (1999).
- C.F. Kao, C.L. Jeng, Ceram. Int. 26, 237, (2000).
- A. Neumann, D. Walter, Thermochim. Acta. 445, 200, (2006).
- M. Mazloumi, N. Shahcheraghi, A. Kajbafval, S. Zanganeh, A. Lak, M.S. Mohajerani, S.K. Sadrinezhaad, J. Alloys Compd. 473, 283, (2009).
- H.E. Zhang, B.F. Zhang, G.F. Wang, X.H. Dong, Y. Gao, J. Magn. Magn. Mater. 312, 126, (2007).
- L.L. Lorentz-Petersen, P. Jensen, R. Madsen, Synthesis, 24, 4110, (2009).
- R. Juday, H. Adkins, J. Am. Chem. Soc. 77, 4559, (1955).
- W. He, L. Wang, C. Sun, K. Wu, S. He, J. Chen, P. Wu, Z. Yu, Chem. Eur. J. 17, 13308, (2011).
- N. Azizi, E. Akbari, A.K. Amiri, M.R. Saidi, Tetrahedron Lett. 49, 6682, (2008).
- H. Goksu, S.F. Ho, O.N. Metin, K. Korkmaz, A.G. Mendoza, M.S. Gultekin, S. Sun, ACS Catal. 4, 1777, (2014).
- A. Galan, J. De Mendoza, P. Prados, J. Rojo, A.M. Echavarren, J. Org. Chem. 56, 452, (1991).


