STRUCTURE OF fac-[(ppyEt)Re(CO)3Br], A MONONUCLEAR ReI COMPLEX DERIVED FROM PYRROLINE-PYRAZOLYL-PYRIDAZINE

- PYRROLINE-PYRAZOLYL-PYRIDAZINE,
- Structure,
- Rhenium (I)
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
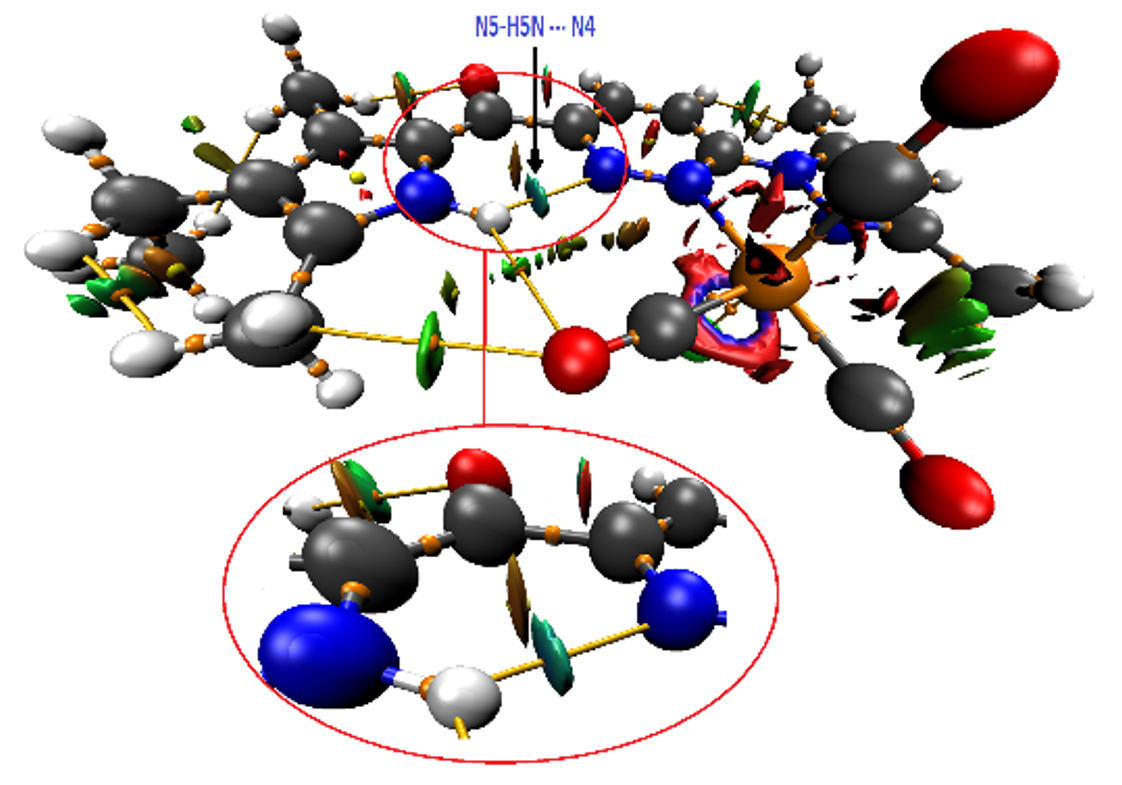
The reaction of 6-(1H-3,5-dimethylpyrazolyl)pyridazine-3-carboxylic acid with oxalyl chloride and then 2,4-dimethyl-3-ethylpyrrole leads to [6-(3,5-dimethyl-1H-pyrazol-1-yl)pyridazin-3-yl](4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl)methanone (ppyEt). The reaction of ppyEt with bromotricarbonyl(tetrahydrofuran)-rhenium(I) dimer leads to [(ppyEt)Re(CO)3Br] compound. The molecule of [(ppyEt)Re(CO)3Br] displays a non-regular octahedron around the rhenium(I) center with a bromide. The molecule defines an intramolecular hydrogen bond between the 4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl hydrogen atom and one nitrogen from the pyridazin-3-yl fragment with D···A 2.833(17) Å. The coplanarity of the 4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl-carbonyl is attributed to this intramolecular bond, in addition to C—H···O≡C and N—H···O≡C interactions as suggested by NCI calculations.
References
- Kumar, A., S.-S. Sun, and A.J. Lees, Photophysics and Photochemistry of Organometallic Rhenium Diimine Complexes, in Photophysics of Organometallics, A.J. Lees, Editor. 2010, Springer: Berlin Heidelberg. p. 37-71.
- Vlček, A., Ultrafast Excited-State Processes in Re(I) Carbonyl-Diimine Complexes: From Excitation to Photochemistry, in Photophysics of Organometallics, J.A. Lees, Editor. 2010, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 115-158.
- Stufkens, D.J. and A. Vlček, Ligand-dependent excited state behaviour of Re(I) and Ru(II) carbonyl–diimine complexes. Coordination Chemistry Reviews, 1998. 177(1): p. 127-179.
- Kiefer, L.M., J.T. King, and K.J. Kubarych, Dynamics of rhenium photocatalysts revealed through ultrafast multidimensional spectroscopy. Acc Chem Res, 2015. 48(4): p. 1123-30.
- Sato, S., T. Arai, and T. Morikawa, Toward Solar-Driven Photocatalytic CO2 Reduction Using Water as an Electron Donor. Inorg Chem, 2015. 54(11): p. 5105-13.
- Oriskovich, T.A., P.S. White, and H.H. Thorp, Luminescent labels for purine nucleobases - electronic-properties of guanine bound to rhenium(I). Inorganic Chemistry, 1995. 34(7): p. 1629-1631.
- Lo, K.K., et al., Rhenium(I) polypyridine biotin isothiocyanate complexes as the first luminescent biotinylation reagents: synthesis, photophysical properties, biological labeling, cytotoxicity, and imaging studies. Inorg Chem, 2008. 47(2): p. 602-11.
- Man, L.L.S. and C.W. Kin, Photosensitizing Properties of Some Rhenium(I) Tricarbonyl Diimine Complexes. ChemPhysChem, 2001. 2(4): p. 252-256.
- Chang, K.-C., et al., Anion recognition and sensing by transition-metal complexes with polarized NH recognition motifs. Coordination Chemistry Reviews, 2015. 284(0): p. 111-123.
- Chang, K.C., S.S. Sun, and A.J. Lees, Anion sensing by rhenium(I) carbonyls with polarized N-H recognition motifs. Inorganica Chimica Acta, 2012. 389(0): p. 16-28.
- Salmain, M., et al., Labeling of proteins by organometallic complexes of rhenium. (I). Synthesis and biological activity of the conjugates. Bioconjug Chem, 1993. 4(6): p. 425-33.
- Santoro, G., et al., Synthesis, characterization and cellular location of cytotoxic constitutional organometallic isomers of rhenium delivered on a cyanocobalmin scaffold. Dalton Trans, 2015. 44(15): p. 6999-7008.
- Wahler, K., et al., Rhenium Complexes with Red-Light-Induced Anticancer Activity. Eur J Inorg Chem, 2014. 2014(5): p. 807-811.
- Ma, D.L., et al., DNA binding and cytotoxicity of ruthenium(II) and rhenium(I) complexes of 2-amino-4-phenylamino-6-(2-pyridyl)-1,3,5-triazine. Inorg Chem, 2007. 46(3): p. 740-9.
- Louie, M.W., et al., Synthesis, Emission Characteristics, Cellular Studies, and Bioconjugation Properties of Luminescent Rhenium(I) Polypyridine Complexes with a Fluorous Pendant. Organometallics, 2012. 31(16): p. 5844-5855.
- Windle, C.D., et al., CO2 photoreduction with long-wavelength light: dyads and monomers of zinc porphyrin and rhenium bipyridine. Chem Commun (Camb), 2012. 48(66): p. 8189-91.
- Windle, C.D., et al., Improving the photocatalytic reduction of CO2 to CO through immobilisation of a molecular Re catalyst on TiO2. Chemistry, 2015. 21(9): p. 3746-54.
- Schneider, T.W. and A.M. Angeles-Boza, Competitive 13C and 18O kinetic isotope effects on CO2 reduction catalyzed by Re(bpy)(CO)3Cl. Dalton Trans, 2015. 44(19): p. 8784-7.
- Franco, F., et al., Photo- and Electrocatalytic Reduction of CO2 by [Re(CO)3{a,a'-diimine-(4-piperidinyl-1,8-naphthalimide)}Cl] Complexes. European Journal of Inorganic Chemistry, 2015(2): p. 296-304.
- Sahara, G. and O. Ishitani, Efficient Photocatalysts for CO2 Reduction. Inorg Chem, 2015. 54(11): p. 5096-104.
- Agarwal, J., et al., Mechanisms for CO production from CO2 using reduced rhenium tricarbonyl catalysts. J Am Chem Soc, 2012. 134(11): p. 5180-6.
- Morimoto, T., et al., CO2 capture by a rhenium(I) complex with the aid of triethanolamine. J Am Chem Soc, 2013. 135(45): p. 16825-8.
- Shakeri, J., H. Hadadzadeh, and H. Tavakol, Photocatalytic reduction of CO2 to CO by a dinuclear carbonyl polypyridyl rhenium(I) complex. Polyhedron, 2014. 78(0): p. 112-122.
- Lees, A.J., Luminescence Properties of Organometallic Complexes. Chemical Reviews, 1987. 87(4): p. 711-743.
- Sacksteder, L., et al., Luminescence studies of pyridine alpha-diimine rhenium(I) tricarbonyl complexes. Inorganic Chemistry, 1990. 29(21): p. 4335-4340.
- Smithback, J.L., et al., Preparative routes to luminescent mixed-ligand rhenium(I) dicarbonyl complexes. Inorg Chem, 2006. 45(5): p. 2163-74.
- Pizarro, N., et al., Dual Emission of a Novel (P,N) ReI Complex: A Computational and Experimental Study on [P,N-{(C6H5)2(C5H4N)P}Re(CO)3Br]. The Journal of Physical Chemistry A, 2015. 119(17): p. 3929-3935.
- Venegas, F., N. Pizarro, and A. Vega, STRUCTURAL AND PHOTOPHYSICAL PROPERTIES OF A MONONUCLEAR Re(I) COMPLEX:[P,N-{(C6H5)2(C5H5N)P}Re(CO)3Br]. Journal of the Chilean Chemical Society, 2011. 56: p. 823-826.
- Pizarro, N., et al., 1IL and 3MLCT Excited States Modulated by H+: Structure and Photophysical Properties of [(2-bromo-5-(1H-pyrazol-1-yl)pyrazine)Re(CO)3Br] Unpublished results, 2018.
- Saldías, M., et al., Mononuclear ReI complexes derived from pyrazolyl-piridazine: Structure and Photophysical Properties. Submitted to ACS Omega 2019: p. 194-201.
- Loudet, A. and K. Burgess, BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chemical Reviews, 2007. 107(11): p. 4891-4932.
- Portenkirchner, E., et al., Electrocatalytic and photocatalytic reduction of carbon dioxide to carbon monoxide using the alkynyl-substituted rhenium(I) complex (5,5′-bisphenylethynyl-2,2′-bipyridyl)Re(CO)3Cl. Journal of Organometallic Chemistry, 2012. 716: p. 19-25.
- SAINT-Plus. 1999.
- Sheldrick, G.M., SHELXL97. 1997.
- Sheldrick, G.M., A short history of SHELX. Acta Crystallogr. A, 2008. 64(Pt 1): p. 112-22.
- Sheldrick, G.M.S.N.V., Bruker AXS Inc., Madison, WI, USA, 2000.
- SADABS. 2004.
- Frisch, M.J.T., G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. , Gaussian 09. 2009, Gaussian, Inc.: Wallingford CT.
- Lu, T. and F. Chen, Multiwfn: A multifunctional wavefunction analyzer. Journal of Computational Chemistry, 2012. 33(5): p. 580-592.
- Humphrey, W., A. Dalke, and K. Schulten, VMD: Visual molecular dynamics. Journal of Molecular Graphics, 1996. 14(1): p. 33-38.
- Johnson, E.R., et al., Revealing Noncovalent Interactions. Journal of the American Chemical Society, 2010. 132(18): p. 6498-6506.
- Nkungli, N.K. and J.N. Ghogomu, Theoretical analysis of the binding of iron(III) protoporphyrin IX to 4-methoxyacetophenone thiosemicarbazone via DFT-D3, MEP, QTAIM, NCI, ELF, and LOL studies. Journal of Molecular Modeling, 2017. 23(7): p. 200.
- Contreras-García, J., et al., A benchmark for the non-covalent interaction (NCI) index or… is it really all in the geometry? Theoretical Chemistry Accounts, 2016. 135(10): p. 242.
- Saleh , G., et al., Revealing Non-covalent Interactions in Molecular Crystals through Their Experimental Electron Densities. Chemistry – A European Journal, 2012. 18(48): p. 15523-15536.
- Belkhiria, M., et al., Synthesis and structural studies of hexafluorophosphate-based organic salts: A combined experimental and computational analysis. Journal of Molecular Structure, 2020. 1202: p. 127337.