IDENTIFICATION OF COEXISTING INDIGO SPECIES IN AN ANCIENT GREEN THREAD USING DIRECT PLASMON-ENHANCED RAMAN SPECTROSCOPY

- Ancient thread,
- Indigo,
- Leuco-Indigo,
- Raman,
- SERS
Copyright (c) 2020 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
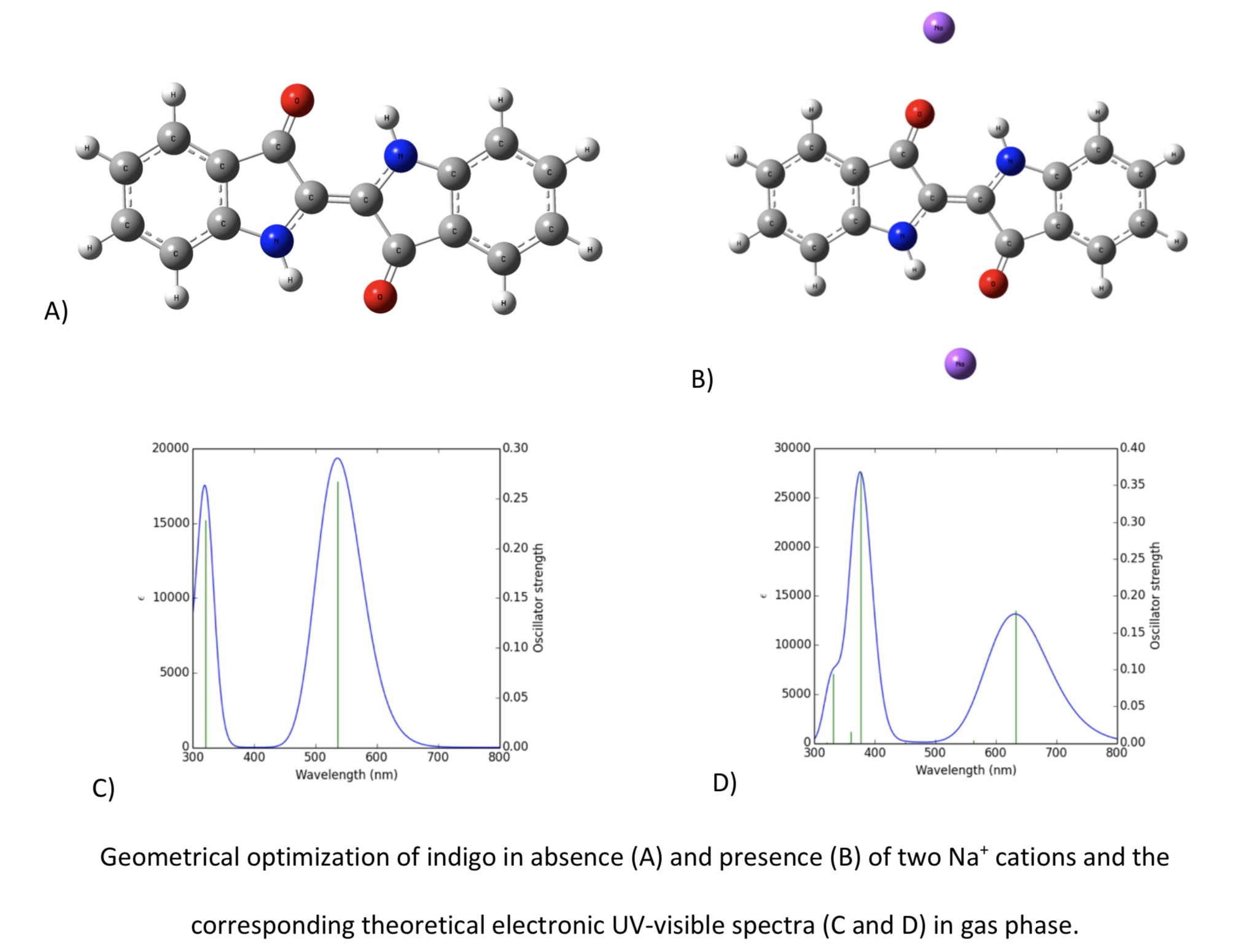
A green ancient thread sample from a Chilean mummy turban was analyzed by plasmon-enhanced Raman scattering spectroscopy using a direct drop-colloidal method. The enhanced-Raman signals in the sample are associated with biomolecules from the thread and two coexisting dyes, indigo and leuco-indigo. The presence of indigo (blue colour) was identified from its most characteristic vibrational bands. Leuco-indigo (yellow colour) was identified for the first time in an ancient textile; its SERS signals are coincident with the SERS bands of a synthesized leuco-indigo. The interconversion leuco-indigo to indigo was followed by UV-visible spectroscopy. Based on theoretical calculations it is proposed that the interconversion involves a electron delocalization mainly around the NC-CN bridge. The mixture of both dyes (indigo and leuco-indigo) is the responsible for the green colour observed.
References
- T. Maugard, E. Enaud, P. Choisy, M. D. Legoy. Phytochemistry. 58, 897–904 (2001).
- W. Maier, B. Schumann, D. Gröger. Phytochemistry. 29, 817–819 (1990).
- J. C. Splitstoser, T. D. Dillehay, J. Wouters, A. Claro. Sci. Adv. 2, e1501623 (2016).
- A. N. Padden, V. M. Dillon, J. Edmonds, M. D. Collins, N. Alvarez, P. John. Int. J. Syst. Bacteriol. 49, 1025–1031 (1999).
- A. Roquero. An. del Mus. América. 3, 145–160 (1995).
- A. N. Padden, V. M. Dillon, P. John, J. Edmonds, M. D. Collins, N. Alvarez. Nature. 396, 225–225 (1998).
- N. Mermod, S. Harayama, K. N. Timmis. Bio/Technology. 4, 321–324 (1986).
- J. Wouters, N. Rosario-Chirinos. J. Am. Inst. Conserv. 31, 237–255 (1992).
- L. Degani, C. Riedo, O. Chiantore. Anal. Bioanal. Chem. 407, 1695–1704 (2015).
- A. Fiedler, M. Baranska, H. Schulz. J. Raman Spectrosc. 42, 551–557 (2011).
- R. Mulholland, D. Howell, A. Beeby, C. E. Nicholson, K. Domoney. Herit. Sci. 5, 1–19 (2017).
- L. H. Oakley, D. M. Fabian, H. E. Mayhew, S. A. Svoboda, K. L. Wustholz. Anal. Chem. 84, 8006–8012 (2012).
- M. Ricci, C. Lofrumento, E. Castellucci, M. Becucci. J. Spectrosc. 2016, 1–10 (2016).
- M. A. García-Bucio, E. Casanova-González, J. L. Ruvalcaba-Sil, E. Arroyo-Lemus, A. Mitrani-Viggiano. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 374, 20160051 (2016).
- H. Schlüter. Textilveredlung. 25, 218–221 (1990).
- A. Roessler, O. Dossenbach, P. Rys. J. Electrochem. Soc. 150, D1 (2003).
- A. Roessler, X. Jin. Dye. Pigment. 59, 223–235 (2003).
- E. Platania, C. Lofrumento, E. Lottini, E. Azzaro, M. Ricci, M. Becucci. Anal. Bioanal. Chem. 407, 6505–6514 (2015).
- O. Otłowska, M. Ślebioda, A. Kot-Wasik, J. Karczewski, M. Śliwka-Kaszyńska, Molecules, 23, 339 (2018).
- G. Focacci. Rehue, 49–63 (1969).
- G. Focacci. Chungara, Rev. Antropol. Chil. 3, 23–74 (1974).
- F. W. Billmeyer, M. Saltzman, Principles of color technology. In: Color and Color Difference Measurement (John Wiley & Sons, Inc., New York, 1981).
- R. S. Hunter, R. W. Harold, J. Wiley, The Measurement of Appearance (John Wiley & Sons, Inc., New York, ed. 2nd, 1987).
- S. Hoces de la Guardia Ch, P. Brugnoli B, P. Jelvez H. Bol. del Mus. Chil. Arte Precolomb. 16, 67–92 (2011).
- F. Celis, M. M. Campos-Vallette, J. S. Gómez-Jeria, R. E. Clavijo, G. P. Jara, C. Garrido. Spectrosc. Lett. 49, 336–342 (2016).
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. M. Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian (2009).
- S. I. Gorelsky, AOMix: Program for Molecular Orbital Analysis (2012), (available at http://www.sg-chem.net/).
- T. Aguayo, C. Garrido, R. E. Clavijo, J. S. Gómez-Jeria, C. Araya Monasterio, M. Icaza, F. Espinoza Moraga, M. M. Campos Vallette. J. Raman Spectrosc. 44, 1238–1245 (2013).
- M. C. Caraher, A. Sophocleous, J. R. Beattie, O. O’Driscoll, N. M. Cummins, O. Brennan, F. J. O’Brien, S. H. Ralston, S. E. J. Bell, M. Towler, A. I. Idris. Biochim. Biophys. Acta - Mol. Basis Dis. 1864, 398–406 (2018).
- A. Kuzuhara. Kobunshi Ronbunshu. 69, 313–325 (2012).
- W. Akhtar, H. G. M. Edwards, D. W. Farwell, M. Nutbrown. Spectrochim Acta Part A. 53, 1021–1031 (1997).
- A. Kuzuhara. Biopolymers. 81, 506–514 (2006).
- F. Madzharova, Z. Heiner, J. Kneipp. J. Phys. Chem. C. 121, 1235–1242 (2017).
- A. Baran, A. Fiedler, H. Schulz, M. Baranska. Anal. Methods. 2, 1372 (2010).
- G. Corales, F. Celis, J. S. Gómez-Jeria, M. Campos, J. J. Cárcamo-Vega. Spectrosc. Lett. 50, 316–321 (2017).
- J. Seixas de Melo, A. P. Moura, M. J. Melo. J. Phys. Chem. A. 108, 6975–6981 (2004).
- M. M. Sousa, C. Miguel, I. Rodrigues, A. J. Parola, F. Pina, J. S. Seixas De Melo, M. J. Melo. Photochem. Photobiol. Sci. 7, 1353–1359 (2008).
- J. B. Weinrach, D. R. Meyer, T. G. J. Joseph, P. E. Michalski, K. L. Carter, D. S. Grubisha, D. W. Bennett. Jorunal Crystallogr. Spectrosc. Res. 22, 291–301 (1992).
- H. Takahashi, N. Kaneko, K. Miwa. Spectrochim. Acta Part A Mol. Spectrosc. 38, 1147–1153 (1982).
- G. Socrates, lnfrared Characteristic Group Frequencies: Tables and Charts (John Wiley & Sons, Ltd, Chichester, ed. 3rd, 2004).
- D. Lin-Vien, N. B. Colthup, W. G. Fateley, J. G. Grasselli, The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules (Academic Press, London, ed. 1st, 1991).
- D. V. Konarev, L. V. Zorina, S. S. Khasanov, A. F. Shestakov, A. M. Fatalov, A. Otsuka, H. Yamochi, H. Kitagawa, R. N. Lyubovskaya. Inorg. Chem. 57, 583–589 (2018).
- K. Fukatsu. Sen’i Gakkaishi. 44, 238–242 (1988).
- D. Balköse, H. Baltacioǧlu. J. Chem. Technol. Biotechnol. 54, 393–397 (1992).

