NEW ROUTE FOR THE SYNTHESES OF ITS SOME NOVEL DERIVATIVES OF 3-ARYL BENZO[D]THIAZOLE-2(3H)-IMINE FROM HIGH SUBSTITUTED THIOUREAS

- 3-Aryl benzo[d]thiazole-2(3H)-imines,
- Diazonium salt,
- N- acyl-N'-aryl thiourea
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
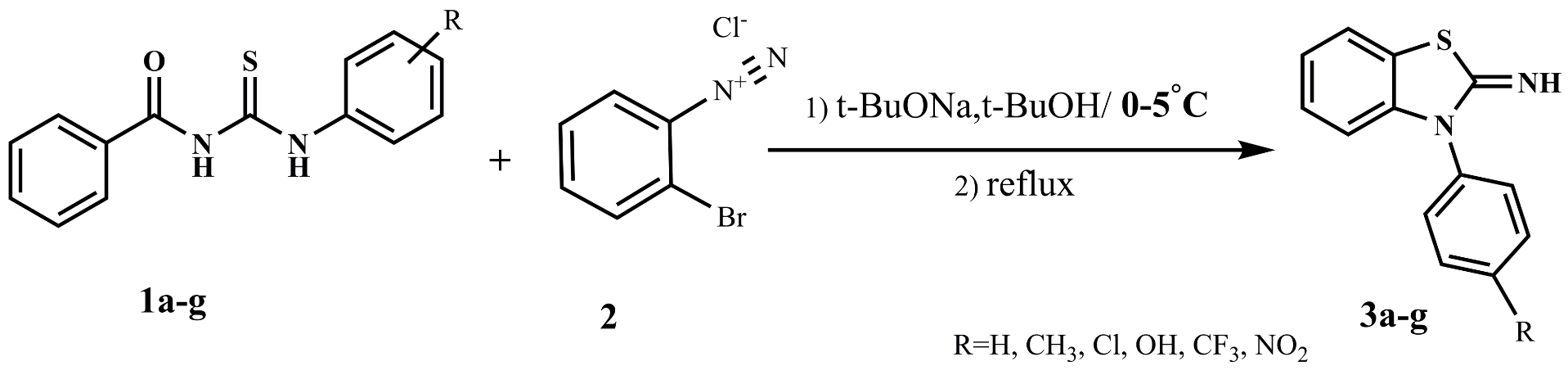
We have developed herein a new approach to diverse synthesis of novel derivatives of 3-Aryl benzo[d]thiazole-2(3H)-imines (3a-g), by a two-component reaction between diazonium salt (2) and various synthesized N-acyl-N'-aryl thioureas (1a-g), in the presence of sodium tert butoxide as strong base. Finally, it resulted in the production of the desired product with a moderate yield. The chemical structures of these synthesized compounds were confirmed by various physico-chemical methods viz. FT-IR, 1H-NMR, 13C-NMR and elemental analysis.
References
- . Sreedevi, R.; Saranya, S.; Rohit, K. R.; Anilkumar, G.; Adv. Synth. Catal. 2019, 361, 2236.
- . Kaur, N.; Bhardwaj, P.; Devi, M., Verma, Y.; Grewal, P.; Synth. Commun. 2019, 49, 1345.
- . Da Costa, L.; Scheers, E.; Coluccia, A.; Rosetti, A.; Roche, M.; Neyts, J.; Terme, T.; Cirilli, R.; Mirabelli, C.; Silvestri, R.; Vanelle, P.; Eur. J. Med. Chem. 2017, 140, 528.
- . Khokra, S. L.; Arora, K.; Khan, S. A.; Kaushik, P.; Saini, R.; Husain, A.; Iran J. Pharm. Res. 2019, 18, 1.
- . Tariq, S.; Kamboj, P.; Amir, M.; Arch. Pharm. (Weinheim). 2019, 352, 1.
- . Diao, P. C.; Lin, W. Y.; Jian, X. E.; Li, Y. H.; You, W. W.; Zhao, P. L.; Eur. J. Med. Chem. 2019, 179, 196.
- . Romeo, G.; Prezzavento, O.; Intagliata, S.; Pittalà, V.; Modica, M. N.; Marrazzo, A.; Turnaturi, R.; Parenti, C.; Chiechio, S.; Arena, E.; Campisi, A.; Sposito, G.; Salerno, L.; Eur. J. Med. Chem. 2019, 174, 226.
- . Firoozpour, L.; Mokhtari, A.; Moghimi, S.; Safavi, M.; Foroumadi, A.; J. Sci. Islam. Repub. Iran 2018, 29, 335.
- . Haroun, M.; Tratrat, C.; Kositsi, K.; Tsolaki, E.; Petrou, A.; Aldhubiab, B.; Attimarad, M.; Harsha, S.; Geronikaki, A.; Venugopala, K. N.; Elsewedy, H. S.; Sokovic, M.; Glamoclija, J.; Ciric, A.; Curr. Top. Med. Chem. 2018, 18, 75.
- . Chhabra, M.; Sinha, S.; Banerjee, S.; Paira, P.; Bioorg. Med. Chem. Lett. 2016, 26, 213.
- . Wu, J.; Luo, H.; Wang, T.; Sun, H.; Zhang, Q.; Chai, Y.; Tetrahedron 2019, 75, 1052.
- . Rabbani, M. G.; Islamoglu, T.; El-Kaderi, H. M.; J. Mater. Chem. A 2017, 5, 258.
- . Rouf, A.; Tanyeli, C.; Eur. J. Med. Chem. 2015, 97, 911.
- . El-Damasy, A. K.; Lee, J. H.; Seo, S. H.; Cho, N. C.; Pae, A. N.; Keum, G.; Eur. J. Med. Chem. 2016, 115, 201.
- . Keri, R. S.; Patil, M. R.; Patil, S. A.; Budagumpi, S.; Eur. J. Med. Chem. 2015, 89, 207.
- . Kumari, B.; Chauhan, K.; Trivedi, J.; Jaiswal, V.; Kanwar, S. S.; Pokharel, Y. R.; ChemistrySelect 2018, 3, 11326.
- . Mishra, V. R.; Ghanavatkar, C. W.; Mali, S. N.; Qureshi, S. L.; Chaudhari, H. K.; Sekar, N.; Comput. Biol. Chem. 2019, 78, 330.
- . Naaz, F.; Srivastava, R.; Singh, A.; Singh, N.; Verma, R.; Singh, V. K.; Bioorg. Med. Chem. 2018, 26, 3414.
- . Graham, J.; Wong, C. E.; Day, J.; McFaddin, E.; Ochsner, U.; Hoang, T.; Young, C. L.; Ribble, W.; DeGroote, M. A.; Jarvis, T.; Sun, X.; Bioorg. Med. Chem. Lett. 2018, 28, 3177.
- . Eshghi, H.; Eshkil, F.; Shokooh Saljooghi, A.; Bakavoli, M.; Org. Chem. Res. 2019, 5, 87.
- . Vicini, P.; Geronikaki, A.; Incerti, M.; Busonera, B.; Poni, G.; Cabras, C. A.; La Colla, P.; Bioorg. Med. Chem. 2003, 11, 4785.
- . Abdelgawad, M. A.; Bakr, R. B.; Omar, H. A.; Bioorg. Chem. 2017, 74, 82.
- . Zeng, H.; Luo, P.; Luo, M.; Ding, H.; Ding, Q.; Tetrahedron 2019, 75, 130472.
- . Yadav, P. S.; Prakash, D.; Senthilkumar, G. P.; Int. J. Pharm. Sci. Drug. Res. 2011, 3, 1.
- . Chugh, B.; Kumar Singh, A.; Thakur, S.; Pani, B.; Kumar Pandey, A.; Lgaz, H.; Chung, I-M.; Ebenso, E. E.; J. Phys. Chem. C, 2019, 123, 22897.
- . Gill, R. K.; Rawal, R. K.; Bariwal, J.; Arch. Pharm. (Weinheim), 2015, 348, 155.
- . Liao, C.; Kim, U. J.; Kannan, K.; Environ. Sci. Technol. 2018, 52, 5007.
- . Ghanbari Pirbasti, F.; Mahmoodi, N.; J. Chin. Chem. Soc. Taip. 2017, 64, 80.
- . Muhammad, S.; Kumar, S.; Koh, J.; Saravanabhavan, M.; Ayub, K.; Chaudhary, M.; Mol. Simul., 2018, 44, 1191.
- . Antypenko, L.; Sadykova, Z.; Shabelnyk, K.; Meyer, F.; Kovalenko, S.; Meyer, V.; Garbe, L‐A.; Steffens, K.; Arch. Pharm. (Weinheim). 2019, 352, 1.
- . Tavakol, H.; Mahmoudi, A.; Ranjbari, M. A.; J. Sulfur Chem. 2019, 40, 113.
- . Pourshamsian, K.; Int. J. Mol. Clin. Microbiol. 2013, 2, 310.
- . Fioresi, F.; Rouleau, A.; Maximova, K.; Vieillard, J.; Boireau, W.; Elie Caille, C.; Soulignac, C.; Zeggari, R.; Clamens, T.; Lesouhaitier, O.; Mofaddel, N.; Le Derf, F.; Mater. Today: Proc. 2019, 6, 340.
- . Filimonov, V. D.; Krasnokutskaya, E. A.; Kassanova, A. Z.; Fedorova, V. A.; Stankevich, K. S.; Naumov, N. G.; Bondarev, A. A.; Kataeva, V. A.; Eur. J. Org. Chem. 2019, 2019, 665.
- . Metwally, M. A.; Bondock, S.; El-Desouky, E. -S. I.; Abdou, M. M.; Am. J. Chem. 2013, 2, 347.
- . Docherty, J. H.; Peng, J.; Dominey, A. P.; Thomas, S. P.; Nat. Chem. 2017, 9, 595.
- . Jeon, J.; Lee, S. Y.; Cheon, C. H.; Adv. Synth. Catal. 2019, 361, 2360.
- . Adelani Alabi, K.; Mosebolatan Jabar, J.; FUNAI J. Sci. Technol. 2017, 3, 85.
- . Marzi, M.; Pourshamsian, K.; Hatamjafari, F.; Shiroudi, A.; Oliaey, A. R.; Russ. J. Bioorg. Chem. 2019, 45, 391.
- . Kazeminejad, Z.; Pourshamsian, K.; Hatamjafari, F.; Shiroudi, A.; Oliaey, A. R.; Russ. J. Org. Chem., 2019, 55, 1609.
- . Yazdanbakhsh, M. R.; Ghanadzadeh, A.; Moradi, E.; J. Mol. Liq. 2007, 136, 165.