- Bidentate Schiff bases,
- Metal(II) Complexes,
- Antibacterial,
- Antifungal and MIC Activity
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
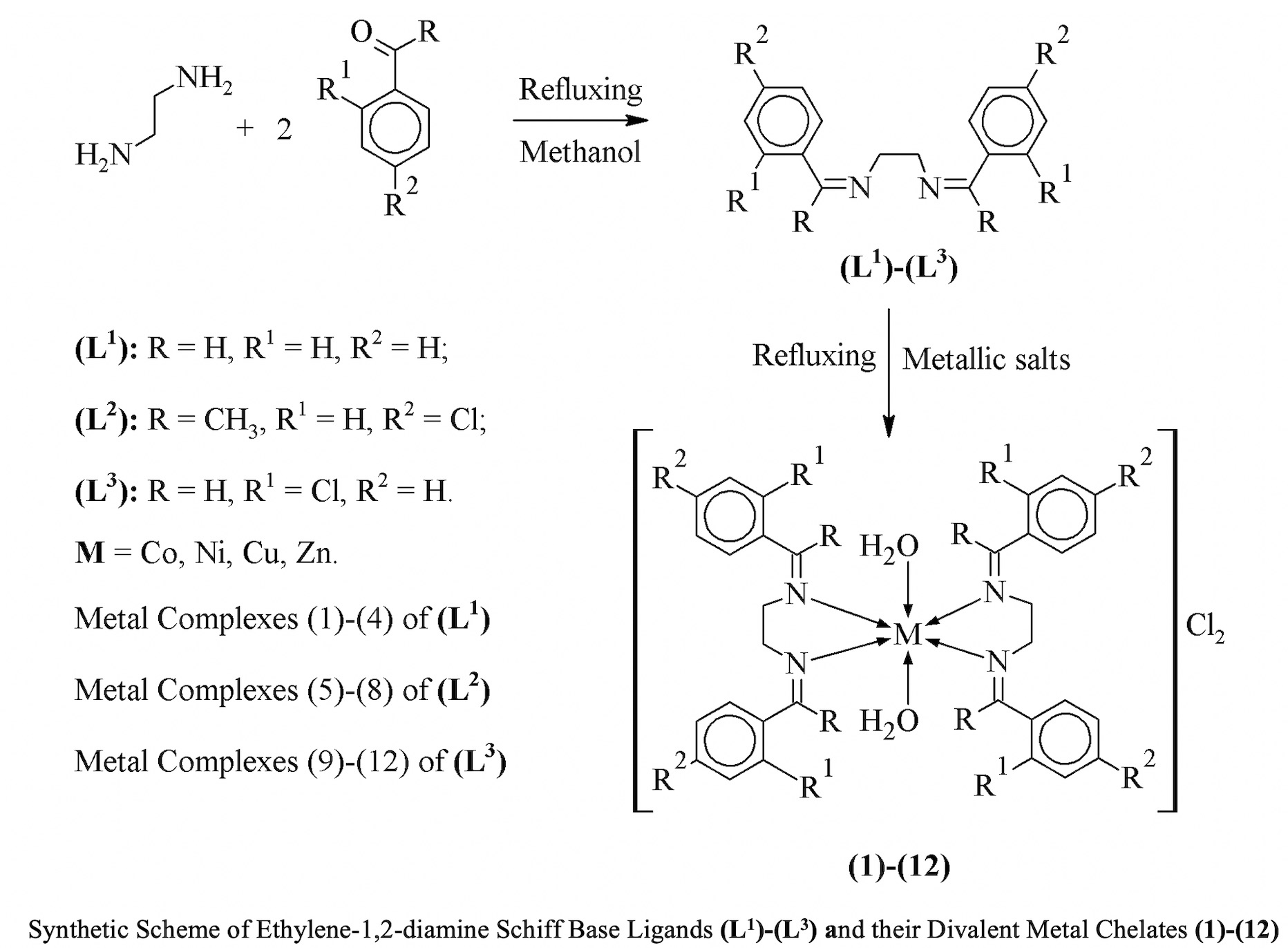
By condensing ethylene-1,2-diamine with different aldehydes such as benzaldehyde, 4-chloroacetophenone and 2-chlorobenzaldeyhde in 1:2 molar ratio a new Schiff base series (L1)-(L3) prepared containing bidentate nitrogen atom. Their metal complexes were synthesized by coordinated the ligand with transition metals as Co(II), Cu(II), Ni(II) and Zn(II). These metal complexes show octahedral geometry. The characterization of the compounds done with the help of spectral, physical and analytical data. Elemental and spectral data of all the ligands and their metal complexes are very consistent with their structure. These show the high purity of these compounds. For in-vitro studies these metal complexes along its ligands were screened against the six bacterial strains; Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Streptococcus faecalis, Klebsiella pneumoniae and Bacillus subtilis. Six fungal strains; Aspergillus niger, Trichophyton mentogrophytes, Epidermophyton floccosum, Trichophyton schoenleinii, Microscopum canis and Fusarium culmorum were used to study antifungal activity of the compounds. One important indication from their antimicrobial activity was that metal complexes showed high activity than the corresponding ligands. Reason behind the increased activity was chelation process that reduces the polarity of metal ion by complexing with bidentate ligands.
References
2. Abd-Elzaher M.-M., J. Chin. Chem. Soc., 2001, vol. 48, p. 153.
3. Chohan, Z.-H., and Hanif, M., J. Enz. Inhib. Med. Chem., 2010, vol. 25, p. 737.
4. Jarrahpour, A.-A., Motamedifar, M., Pakshir, K., Hadi, N., and Zarei, M., Mol., 2004, vol. 9, p. 815.
5. Kabeer, S.-A., Baseer, M.-A., and Mote, N.-A., Asian J. Chem., 2001, vol. 13, p. 496.
6. El-Masry, A.-H., Fahmy, H.-H., and Abdelwahed, S.-H.-A.,, Mol., 2000, vol. 5, p. 1429.
7. Sasikala, K., and Arunachalam, S., Chem. Sci. Trans., 2013, vol. 2(S1), p. S157.
8. Badwaik, V.-B., and Aswar, A.-S., Russ. J. Coord. Chem., 2007, vol. 33, p. 755.
9. Gwaram, N.-S., Ali, H.-M., Khaledi, H. and Abdulla, M.-A., Mol., 2012, vol. 17, p. 5952.
10. Tavman, A., Çinarli, A., Gürbüz, D., and Tan, A.-S.-B., J. Chin. Chem. Soc., 2014, vol. 61, p. 1377.
11. Sumrra, S.-H., Ibrahim, M., Ambreen, S., Imran, M., Danish, M., and Rehmani, F.-S., Bioinorg. Chem. App., 2014, vol. 2014, p. 1. doi 10.1016/j.bmc.2014.08.004
12. Bagihalli, G.-B., Badami, P.-S., and Patil, S.-A., J. Enz. Inhib. Med. Chem., 2009, vol. 24, p. 381. doi org/10.1080/14756360802187901
13. Mohamed, M.-S., Kamel, M.-M., Kassem, E.-M., Abotaleb, N., El-moez, S.-I.-A., and Ahmed, M.-F., Eur. J. Med. Chem., 2010, vol. 45 no. 8, p. 3311.
14. Kabeer, S.-A., Baseer, M.-A., and Mote, N.-A.,, Asian J. Chem., 2001, vol. 13, p. 496.
15. Arion, V.-B., Reisner, E., Fremuth, M., Jokupec, M.-A., Keppler, B.-K., Kukushkin, V.-Y., A., and Pombeiro, J.-L., Inorg. Chem., 2003, vol. 42, p. 6024.
16. Lazic, J.-M., Vucicevic, L., Grguric-Sipka S., et al., , Chem. Med. Chem., 2010, vol. 5, p. 881.
17. Hussein, M.-A., Shaker, R.-M., Ameen, M.-A., and Mohammed, M.-F., Arch. Pharm. Res., 2011, vol. 34, p. 1239.
18. Cui, X.-S., Jing, C., Chai, K.-Y., Lee, J.-S., and Quan, Z.-S., Med. Chem. Res., 2009, vol. 18, p. 49.
19. Kritsanida, M., Mouroutsou, A., Marakos, P., Pouli, N., Papakonstantinou-Garoufalias, S., Pannecouque, C., et al., , Farmaco, 2002, vol. 57, p. 253.
20. Manfredini, S., Vicentini, C.-B., Manfrini, M. Bianchi, N., Rutigliano, C., Mischiati, C., and Gambari, R., Bioorg. Med. Chem., 2000, vol. 8, p. 2343.
21. Turan-Zitouni, G., Kaplancikli, Z.-A., Erol, K., and Kilic, F.-S., Farmaco, 1999, vol. 54, p. 218.
22. Singh, N.-P., and Srivastava, A.-N., J. Serb. Chem. Soc., 2012, vol. 77, p. 627.
23. Shiekh, R.-A., Rahman, I.-A., Malik, M.-A., Masudi, S.-M., and Luddin, N., Internat. J. Electrochem. Sci., 2012, vol. 7, p. 12829.
24. Schertl, S., Hartmann, R.-W., Batzl-Hartmann, C., Bernhardt, G., Sprub, T., Beckenlehner, K., Koch, M., Krauser, R., Schlemmer, R., Gust, R., Schönenberger, H., Arch. der Pharm., 2004, vol. 337, p. 335.
25. Nagababu, P., Latha, J.-N., and Pallavi. et al., , Canad. J. Microb., 2006, vol. 52, p. 1247.
26. Tripath, I.-P., Kumar, M.-M., Ruchita, T., Chinmayi, M., Arti, K., Laxmikant, S., Atul, D., Kumar, S.-U., and Bhihari, P.-K., Res. J. Chem. Sci., 2013, vol. 3, no. 12, p. 54.
27. Rahman, A.-U., Choudhary, M.-I., and Thomsen, W.-J., “Bioassay techniques for drug development”, Harwood Academic, The Netherlands 14, 2001.
28. McLaughlin, J.-L., Chang, C.-J., and Smith, D.-L., “Bench Top” bioassays for the discovery of bioactive natural products: an update, structure and chemistry (part-B),” in Studies in Natural Products Chemistry, Atta-ur-Rahman, Ed., Elsevier Science, Amsterdam, The Netherlands, 1991, vol 9, p. 383.
29. Chohan, Z.-H., and Sumrra, S.-H., Appl. Organomet. Chem., 2010, vol. 24, p. 122.
30. Sumrra, S.-H., and Chohan, Z.-H., Med. Chem. Res., 2013, vol. 22, p. 3934.
31. Sumrra, S.-H., and Chohan, Z.-H., Spectroch. Acta Part A: Mol. Biomol. Spectr., 2012, vol. 98, p. 53.
32. Hanif, M., and Chohan, Z.-H., Spectroch. Acta Part A: Mol. Biomol. Spectr., 2013, vol. 104, p. 468. doi 10.1080/14756360500131911
33. Nyquist. R.-A., Interpreting Infrared, Raman and Nuclear Magnetic Resonance Spectra, Orlando, Academic Press, 2001.
34. Levitt, M., Spin Dynamics: Basics of Nuclear Magnetic Resonance. John Wiley and Sons, 2001.
35. Liu, J., Wu, B., Zhang, B., Liu, Y., Turk. J. Chem., 2006, vol. 30, p. 41.
36. Sarkar, S., and Dey, K., Spectroch. Acta Part A: Mol. Biomol. Spectr., 2005, vol. 62, p. 383.
37. Serbest, K., Kayi, H., Mustafa, E., Sancak, K., Heteroatom Chem., 2008, vol. 19, p. 700.
38. El-Shazly, R.-M., Al-Hazmi, G.-A.-A., Ghazya, S.-E., El-Shahawi, M.-S., and El-Asmya, A.-A., Spectroch. Acta Part A: Mol. Biomol. Spectr., 2005, vol. 61, p. 243.
39. Chandra, S., and Gupta, L.-K., Spectroch. Acta Part A: Mol. Biomol. Spectr., 2005, vol. 61, p. 269.
40. Temel, H., Cakir, U., Otludil, B., and Ugras, H.-G., Synth. React. Inorg. Metal-Org. Nano-Met. Chem., 2001, vol. 31, no. 8, p. 1323.
41. Pavia, D.-L., Lampman, G.-M., Kriz, G.-S., and Vyvyan, J.-R, Spectroscopy, Crooks/Cole, 2007.
42. Estes, W.-E., Gavel, D.-P., Hatfield, W.-B., and Hodgson, D., Inorganic Chemistry, 1978, vol. 17, p. 1415. Doi.org/10.1080/14756360802187901


