- lamotrigine antiepileptics; human serum; liquid chromatography
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
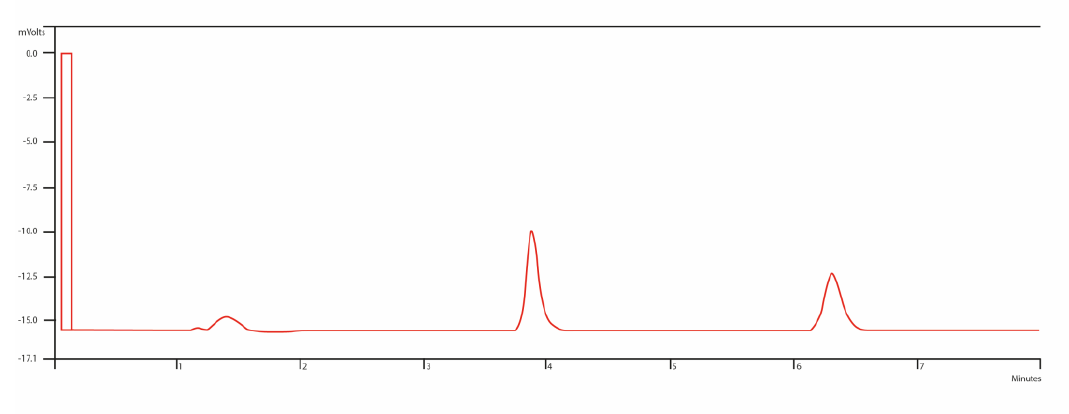
A liquid chromatographic (LC) method for quantitative analysis of lamotrigine in human serum was developed using liquid –liquid extraction with ethyl acetate. Quantitation was achieved over the concentration range of 1.0 to 40.0 µg/mL (r=0.999), using a mixture of acetonitrile: phosphate buffer (0.5 M):(69:31 v/v) as mobile phase, with a flow of 1 mL min−1. Column was C18 (150 mm x 4.6 mm, 5 cm; Merck), chloramphenicol was used as internal standard, and UV detection at α = 306 nm. The intra-assay variation was between 1.22% and 1.85% and the inter-assay was between 1.72% and 2.91%. The detection limit was 0.14 µg/mL, and the quantification limit was 0.42 µg/mL. The method proved to be accurate, with a recovery between 94.02% and 109.95 %, with a RSD not higher than 2.91 % and was selective for lamotrigine (Rs between lamotrigine and chloramphenicol vas 4.9). This method was successfully applied to quantify clozapine in patient serum samples.
In conclusion, the method is precise, accurate, reproducible and selective for the analysis of lamotrigine in human serum. Therefore, it could be an important tool to evaluate drug level in this matrix and, of this way, to obtain a better drug effects.
References
- M, Williams. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals..Merck&Co.Inc, New Jersey, 2013.
- Hardman, J. Limbird, L. Las Bases Farmacológicas de la Terapéutica. Mc Graw-Hill, México, 2006.
- Mc.Evoy, G. AHFS Drug Information. Ed. Bethesda, American Society of Health- System Pharmacists, Rockwile, USA, 2015.
- Sweetman, S. Martindale, Guía Completa de Consulta Farmacoterapéutica. Pharma Editores S.L, Barcelona, 2006.
- W. Froscher, F.Keller, H. Vogt, G. Kramer, Epileptic Disord.4,49–56, (2002).
- B.B. Brzakovic, S.D. Vezmar Kovacevic, K.M. Vucicevic, et al.,J Clin Pharm Ther. 37,693–7, (2012)..
- A.O, Baldoni, P. Freitas‐Lima P, F.I. de Santi Ferreira, et al., Clin Exp Pharmacol Physiol. 43, 685–9, (2016).
- L. Liu, L, Zhao,Q. Wang, F. Qiu, X. Wu, Y. Ma, Eur J Clin Pharmacol. 71,1341–7, (2015).
- K. Inoue, Y. Yamamoto, E. Suzuki, et al,. Eur J Clin Pharmacol.72, 555–62, (2016).
- Q. Wang, M. Liang, Y. Dong, et al., Drug Metab Pharmacokinet.30, 209–13, (2015).
- Y.Yamamoto, Y. Inoue, K. Matsuda, Y. Takahashi, Y. Kagawa, Biol Pharm Bull.35,:487–93, (2012).
- Y. Yamamoto, Y. Takahashi, K. Imai, et al., Drug Metab Pharmacokinet. 30, 214–20, (2015).
- K.R. Kaufman, Bipolar Disord.12, :446–9,( 2010)..
- K.S. Smetana, A.M. Cook, M.L. Bastin, D.R. Oyler, J Crit Care.36, 116–24, (2016).
- P.Douglas-Hall, D. Olubanke, F Gaughran, A. Bile, D.Taylor, Therapeutic Advances in Psycopharmacology, 7, 17-24, (2016).
- O. Beck, I. Ohman, H.K. Nordgren, Ther Drug Monit. 28, 603-7,(2006).
- E Vidal, C Pascual, L Pou, J. .Chromatogr.. B. 736, 295-8, (1999).
- M. Guo, L. Shao,Y. .Du, Anal. Bioana.l Chem.411, 15,(2019):
- S. Itabashi, R. Bito, M. Nishina, et al., Neuropsychopharmacology reports, 39, 48-55, (2019).
- E. Gaïes, M. Bouhlel, R. Charfi, et al.,International Journal Of
- Pharmaceutical Sciences And Research, 2018.
- L. Antonill,V. Brusadin, F.Filipponi, J. Pharm. Biomed. Anal. 56, 763-770, (2011).
- S. Mennickent, R. Fierro, M. Vega, M. de Diego, C.G. Godoy,. JPC 24,, 222-226, (2011).
- F.M. Kerton, R.Marriott, Royal Society of chemistry. 20, (2013)..
- Bioanalytical method Validation. Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration, USA, 2018.