- Alternaric acid,
- Natural products,
- Anti-fungal,
- Phyto-toxic,
- Alternaria solani
Copyright (c) 2017 Sajjad Ahmad, Muhammad Yousaf, Ameer Fawad Zahoor, Matloob Ahmad, Zulfiqar Ali khan, Muhammad Arslan Rashid

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
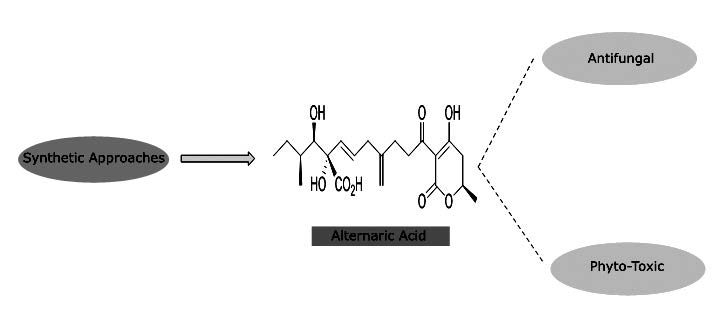
Alternaric acid is characterized as 1,4-diene, possessing (E)-1,2-disubstituted alkene known for nanomolar phytotoxic and antifungal activities. This article highlights the major synthetic approaches attempted towards the total synthesis of alternaric acid reported by three groups (Ichihara & coworkers, Trost & coworkers and Johnson and Slade) which involved novel pathways for the rapid construction of this functionalized natural molecule.
References
- P. W. Brian, P. J. Curtis, H. G. Hemming, C. H. Unwin and J. M. Wright, Nature, 164, 534 (1949).
- P. W. Brian, P. J. Curtis, H. G. Hemming, E. G. Jefferys, C. H. Unwin and J. M. Wright, J. Gen. Microbiol., 5, 619 (1951).
- P. W. Brian, G. W. Elson, H. G. Hemming and J. M. Wright, Ann. Appl. Biol., 39, 308 (1952).
- J. F. Grove, J. Chem. Soc. , 4056 (1952).
- N. Furuichi, S. Nishimura and G. Langsdorf, Ann. Phytopathol. Soc. Jpn., 58, 1 (1992).
- J. R. Bartels-Keith, J. Chem. Soc., 860 (1960).
- J. R. Bartels-Keith, J. Chem. Soc., 1662 (1960).
- H. Tabuchi, H. Oikawa and A. Ichihara, J. Chem. Soc. Perkin Trans.1, 2833 (1994).
- H. Tabuchi, T. Hamamoto, S. Miki, T. Tejima and A. Ichihara, J. Org. Chem., 59, 4749 (1994).
- A. J. Mancuso, S.-L. Huang and D. Swern, J. Org. Chem., 43, 2480 (1978).
- A. Ichihara, Synthesis, 1987, 207 (1987).
- J. C. Fiaud and J. L. Malleron, Tetrahedron Lett., 21, 4437 (1980).
- A. G. Brook, Acc. Chem. Res., 7, 77 (1974).
- I. Nakagawa and T. Hata, Tetrahedron Lett., 16, 1409 (1975).
- M. P. Edwards, S. V. Ley, S. G. Lister, B. D. Palmer and D. J. Williams, J. Org. Chem., 49, 3503 (1984).
- P.-F. Deschenaux, T. Kallimopoulos, H. Stoeckli-Evans and A. Jacot- Guillarmod, Helv. Chim. Acta, 72, 731 (1989).
- M. Julia and J.-M. Paris, Tetrahedron Lett., 14, 4833 (1973).
- Y. Tanabe, M. Miyakado, N. Ohno and H. Yoshioka, Chem. Lett., 11, 1543 (1982).
- H. Tabuchi, T. Hamamoto and A. Ichihara, Synlett, 1993, 651 (1993).
- T. Ohe, N. Miyaura and A. Suzuki, J. Org. Chem., 58, 2201 (1993).
- G. D. P. Barry M. Trost, and Andreas Schoop, J. Am. Chem. Soc., 9228 (1998).
- R. S. Atkinson and M. J. Grimshire, J. Chem. Soc. Perkin Transactions 1, 1215 (1986).
- P. L. Anelli, Montanari F., Quici S, Org. Synth., 69, 212 (1990).
- J. K. Stille, Angew. Chem., Int. Ed. Engl., 25, 508 (1986).
- H. C. Kolb, M. S. VanNieuwenhze and K. B. Sharpless, Chem. Rev., 94, 2483 (1994).
- P. G. Andersson and K. B. Sharpless, J. Am. Chem. Soc., 115, 7047 (1993).
- P. A. Grieco, S. Gilman and M. Nishizawa, J. Org. Chem., 41, 1485 (1976).
- M. C. Slade and J. S. Johnson, Beilstein J. Org. Chem., 9, 166 (2013).
- P. R. Blakemore, J. Chem. Soc. Perkin Transactions 1, 2563 (2002).
- T. Gaich and P. S. Baran, J. Org. Chem., 75, 4657 (2010).
- H. Kim, J. B. Baker, S.-U. Lee, Y. Park, K. L. Bolduc, H.-B. Park, M. G. Dickens, D.-S. Lee, Y. Kim, S. H. Kim and J. Hong, J. Am. Chem. Soc., 131, 3192 (2009).
- D. E. Ward and A. Kazemeini, J. Org. Chem., 77, 10789 (2012).
- K.-M. Chen, K. G. Gunderson, G. E. Hardtmann, K. Prasad, O. Repic and M. J. Shapiro, Chem. Lett., 16, 1923 (1987).
- J.-L. Renaud, C. Aubert and M. Malacria, Tetrahedron Lett., 40, 5015 (1999).
- H. A. Reichard, J. C. Rieger and G. C. Micalizio, Angew. Chem., Int. Ed., 47, 7837 (2008).
- H. Ren, A. Krasovskiy and P. Knochel, Org. Lett., 6, 4215 (2004).


