
- etoricoxib,
- activity,
- DFT,
- COX-2,
- docking
Copyright (c) 2020 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
In this work, a computational chemical study of Etoricoxib was carried out at the B3LYP/6311G(d,p) level of theory, at the gas, aqueous and ethanol phases. Through the chemical reactivity descriptors derived from the DFT, it was possible to find that Etoricoxib structure exhibits a major chemical activity in water and ethanol phases in comparison to the gas phase, which suggests this drug would be more active in biological solvents like in blood, tissues and places where the ciclooxigenasa 2 (COX)-2 is found. In addition, a molecular docking analysis was conducted to study the interaction of Etoricoxib with the COX-2 active site. The results suggest that Etoricoxib interacts with 19 amino acid residues inside the COX-2 active site.
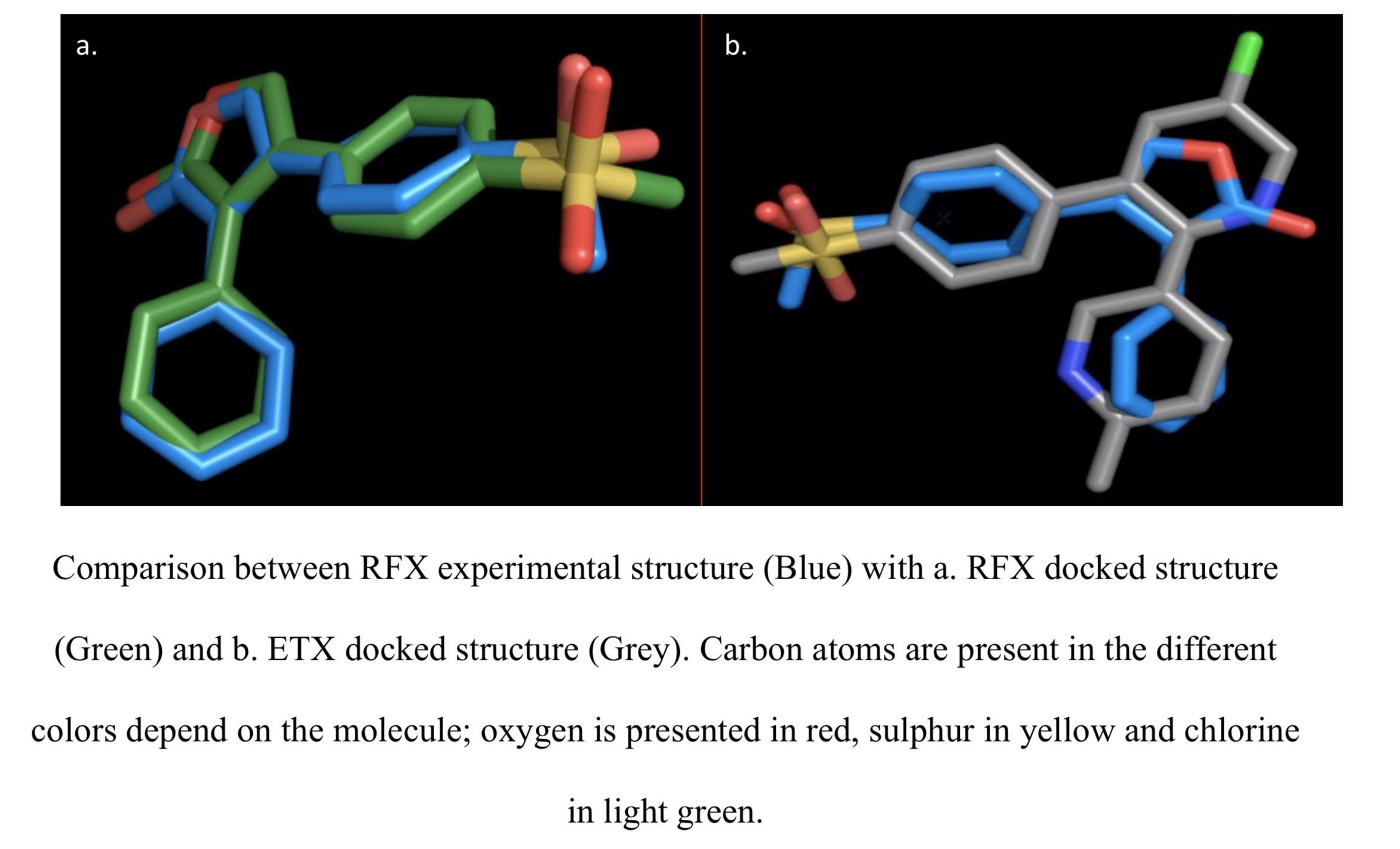
References
- Y. Chai, L. Wang, Y. Bao, R. Teng, Y. Liu, C. Xie, Cryst. Growth Des. 19, 1660, (2019)
- A. Okumu, M. DiMaso, R. Löbenberg, Eur. J. Pharm. Biopharm. 72(1), 91, (2009)
- M. Palucki, Z. Lin, Y. Sun, Org. Process Res. Dev. 9(2), 141, (2005)
- M. Rams-Baron, J. Pacult, A. Jedrzejowska, J. Knapik-Kowalczuk, M. Paluch, Mol. Pharm., 15(9), 3969, (2018)
- H. S. Omer Erkan Yapca, Mehmet Ibrahim Turan, Ismayil Yilmaz, Suleyman Salman, Mine Gulapoglu, J. Obstet. Gynaecol. Res. 40(6), 1674, (2014)
- M. Rams-Baron, Z. Wojnarowska, K. Grzybowska, M. Dulski, J. Knapik, K. Jurkiewicz, W. Smolka, W. Sawicki, A. Ratuszna, M. Paluch, Mol. Pharm. 12(10), 3628, (2015)
- T. Zhang, L. Wang, Y. Bao, Q. Yang, L. Zhou, H. Hao, C. Xie, J. Pharm. Sci. 107(7), 1903, (2018)
- P. Grobelny, A. Mukherjee, G. R. Desiraju, CrystEngComm. 14, 5785, (2012)
- C. Rodríguez-Tinoco, M. Rams-Baron, K. L. Ngai, K. Jurkiewicz, J. Rodríguez-Viejo, M. Paluch, Phys. Chem. Chem. Phys. 20, 3939, (2018)
- N. G. B. Agrawal, A. G. Porras, C. Z. Matthews, M. J. Rose, E. J. Woolf, B. J. Musser, A. L. Dynder, K. E. Mazina, K. C. Lasseter, T. L. Hunt, et al., J. Clin. Pharmacol. 43(3), 268, (2003)
- S. Oniga, L. Pacureanu, C. Stoica, M. Palage, A. Crăciun, L. Rusu, E.-L. Crisan, C. Araniciu, Molecules 22(9), 1507, (2017)
- S. Sil, T. Ghosh, J. Neuroimmunol. 317, 15, (2018)
- T. Yapanoglu, F. Ozkaya, A. H. Yilmaz, R. Mammadov, F. K. Cimen, E. Hirik, Korean J. Physiol. Pharmacol. 21(5), 457, (2017)
- A. Maheshwari, L. Badgujar, B. Phukan, S. L. Bodhankar, P. Thakurdesai, Eur. J. Pharmacol. 667(1-3), 230, (2011)
- K. Anbazhakan, K. Sadasivam, R. Praveena, Struct. Chem. 30(4), 1, (2019)
- M. Leopoldini, T. Marino, N. Russo, M. Toscano, J. Phys. Chem. A. 108(2), 4916, (2004)
- R. Praveena, K. Sadasivam, V. Deepha, R. Sivakumar, J. Mol. Struct. 1061(5), 114, (2014)
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, et al., (2009)
- P. Geerlings, F. De Proft, W. Langenaeker, Chem. Rev. 103(5), 1793, (2003)
- R. G. Parr, W. Yang, Density-Functional Theory of Atoms and Molecules, First ed., Oxford University Press, New York, 1989.
- R. G. Parr, R. G. Pearson, J. Am. Chem. Soc. 105(26), 7512, (1983)
- R. G. Pearson, J. Chem. Educ. 64(7), 561, (1987)
- R. G. Parr, P. K. Chattaraj, J. Am. Chem. Soc. 113(5), 1854, (1991)
- R. G. Pearson, J. Am. Chem. Soc. 107(24), 6801, (1985)
- PyMOL, The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.
- E. F. Pettersen, T. D. Goddard, C. C. Huang, G. S. Couch, D. M. Greenblatt, E. C. Meng, T. E. Ferrin, J. Comput. Chem. 25(13), 1605, (2004)
- C. Steffen, K. Thomas, U. Huniar, A. Hellweg, O. Rubner, A. Schroer, J. Comput. Chem. 31(16), 2967, (2010)
- D. S. Wishart, Y. D. Feunang, A. C. Guo, E. J. Lo, A. Marcu, J. R. Grant, T. Sajed, D. Johnson, C. Li, Z. Sayeeda, et al., Nucleic Acids Res. 46(D1), D1074, (2018)
- A. J. O. Oleg Trott, A. Schroer, J. Comput. Chem. 31(2), 455, (2010)
- R. A. Laskowski, M. B. Swindells, J. Chem. Inf. Model. 51(10), 2778, (2011)
- D. L. DeWitt, Mol. Pharmacol. 55(4), 625, (1999)
- A. L. Blobaum, L. J. Marnett, J. Med. Chem. 50(7), 1425, (2007)
- R. Ferreira De Freitas, M. Schapira, Medchemcomm. 8, 1970, (2017)
- J. L. Medina-Franco, O. Méndez-Lucio, K. Martinez-Mayorga, Adv. Protein Chem. Struct. Biol. 96, 1, (2014)
- J. E. Donald, D. W. Kulp, W. F. DeGrado, Proteins Struct. Funct. Bioinforma. 79(3), 898, (2011)
- L. Yang, J. Zhang, X. Che, Y. Q. Gao, Methods Enzymol. 578, 169, (2016)

