REMOVAL OF REACTIVE RED 198 FROM AQUEOUS SOLUTIONS USING MODIFIED CLAY: OPTIMIZATION, KINETIC AND ISOTHERM

- Reactive Red 198; modified clay; cetyltrimethylammonium bromide; adsorption isotherm; reaction kinetic
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
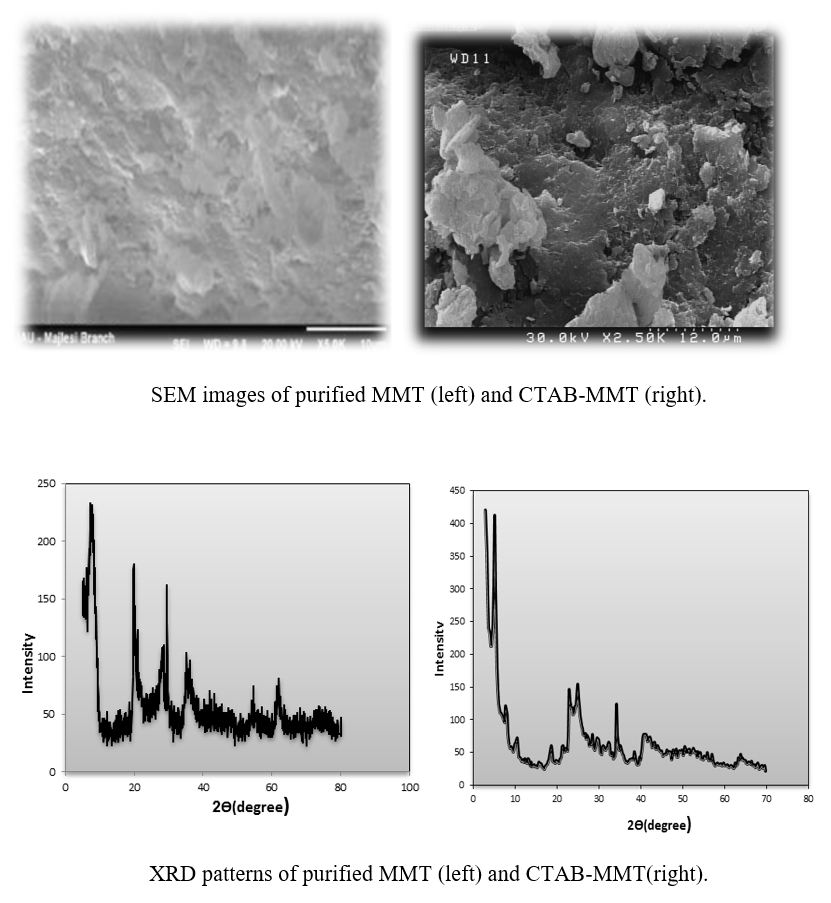
Dyes are large group of environmental pollutions with complex molecular structure and resistant to biodegradation. The entrance of these compounds into the water sources causes health problems in humans and many aquatic organisms. Therefore, colored effluents have to be adequately treated before discharging into the environment. This study aimed to evaluated efficiency of adsorption process of modified clay by cetyltrimethylammonium bromide (CTAB) in removal of Reactive Red 198 (RR 198) from aqueous solution. The modified clay was prepared and its characterization was accomplished by Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) and scanning electron microscopy (SEM). The influences of major operational parameters on the efficiency of adsorption process were investigated and optimized. Kinetic and isotherm of adsorption were determined. The results revealed that increasing pH, initial concentration, ionic strength and temperature decrease rate of dye removal and increasing adsorbent dosage increases the dye removal efficiency. The maximum removal efficiency (99.61%) of RR 198 with modified clay was at pH; 3, time; 60 min, adsorbent dosage; 0.1 g/L and initial concentration; 20 mg/L. The experimental data fitted well to the Langmuir isotherm model and exhibited a maximum adsorption capacity (qmax) of 25.84 mg/g, which followed the pseudo-second-order equation. The adsorption process shows an intra-particle diffusion mechanism. According to the results, modified montmorillonite clay can be considered as an effective and promising adsorbent for the removal of RR 198 from aqueous solutions.
References
- P. Zhang, Q. An, J. Guo, C.C. Wang, J. Colloid Interface Sci. 389, 10, (2013)
- W. Cheah, S. Hosseini, M.A. Khan, T. Chuah, T.S. Choong, Chem. Eng. J. 215, 747, (2013)
- E. Bazrafshan, A.A. Zarei, H. Nadi, M.A. Zazouli, Indian J. Chem. Technol. 21, 105, (2014)
- M. Dehghani, M.M. Taghizadeh, T. Gholami, M. Ghadami, L. Keshtgar, Z. Elhameyan, M.R. Javaheri, N. Shamsedini, F. Jamshidi, S. Shahsavani, M. Ghanbarian, Jundishapur J. Health Sci. 7, 38, (2015)
- M. Alizadeh, E. Bazrafshan, A.H. Mahvi, F. Kord Mostafapour, E. Ghahremani, Sci. J. Kurdistan Univ. Med. Sci. 19, 124, (2014)
- M.A. Zazouli, D. Balarak, Y. Mahdavi, M. Ebrahimi, Iran J. Health Sci. 1, 36, (2013)
- M. Hadi, M.R. Samarghandi, G. McKay, Chem. Eng. J. 160, 408, (2010)
- A. Esmaeili, M. Kalantari, Desalination Water Treat. 57, 6401, (2016)
- K. Nadafi, M. Vosoughi, A. Asadi, M. Omidvar Borna, M. Shirmardi, J. Water Chem. Technol. 36, 125 (2014)
- D. Ghemati, D. Aliouche, J. Water Chem. Technol. 36, 265, (2014)
- H. Biglari, S. Rodríguezí Couto, Y.O. Khaniabadi, H. Nourmoradi, M. Khoshgoftar, A. Amrane, M. Vosoughi, S. Esmaeili, R. Heydari, M.J. Mohammadi, R. Rashidi, Int. J. Chem. React. Eng. 16, 20170064, (2018)
- Y.O. Khaniabadi, R. Heydari, H. Nourmoradi, H. Basiri, H. Basiri, J. Taiwan Inst. Chem. Eng. 68, 90, (2016)
- H. Godini, A. Sheikhmohammadi, L. Abbaspour, R. Heydari, G.S. Khorramabadi, M. Sardar, Z. Mahmoudi, Optik 182, 1194 (2019)
- T. Ahmadifard, R. Heydari, M.J. Tarrahi, G.S. Khorramabadi, Int. J. Chem. React. Eng. 17, 20180154, (2019)
- B. Kamarehie, F. Ahmadi, F. Hafezi, A. Abbariki, R. Heydari, M.A. Karami, Data Brief 18, 96, (2018)
- S.S. Stavitskaya, V.M. Vikarchuk, M.F. Kovtun, O.I. Poddubnaya, A.M. Puziy, J. Water Chem. Technol. 36, 110, (2014)
- N.A. Klymenko, G.M. Zdorovenko, I.A. Shevchuk, L.R. Reshetniak, I.Y. Roi, L.K. Patiuk, J. Water Chem. Technol. 35, 43, (2013)
- M.T. Uddin, M. Rukanuzzaman, M.M.R. Khan, M.A. Islam, J. Environ. Manage. 90, 3443, (2009)
- L. Wang, A. Wang, J. Hazard. Mater. 160, 173, (2008)
- M. Huskić, M. Žigon, M. Ivanković, Appl. Clay Sci. 85, 109, (2013)
- Toor, M. and Jin, B., Chem. Eng. J. 187, 79, (2012)
- J. Ma, J. Qi, C. Yao, B. Cui, T. Zhang, D. Li, Chem. Eng. J. 200, 97, (2012)
- X. Xin, W. Si, Z. Yao, R. Feng, B. Du, L. Yan, Q. Wei, J. Colloid Interface Sci. 359, 499, (2011)
- L. Zhirong, M.A. Uddin, S. Zhanxue, Spectrochim. Acta, Part A 79, 1013 (2011)
- M.F. Hou, C.X. Ma, W.D. Zhang, X.Y. Tang, Y.N. Fan, H.F. Wan, J. Hazard. Mater. 186, 1118, (2011)
- Ö. Gök, A.S. Özcan, A. Özcan, Appl. Surf. Sci. 256, 5439, (2010)
- A. Tabak, N. Baltas, B. Afsin, M. Emirik, B. Caglar, E. Eren, J. Chem. Technol. Biotechnol. 85, 1199, (2010)
- Z. Aksu, A.B. Akın, Chem. Eng. J. 165, 184 (2010)
- G.Q. Wu, X. Zhang, H. Hui, J. Yan, Q.S. Zhang, J.L. Wan, Y. Dai, Chem. Eng. J. 185, 201, (2012)
- A.A. Jalil, S. Triwahyono, S.H. Adam, N.D. Rahim, M.A.A. Aziz, N.H.H. Hairom, N,A,M, Razali, M.A.Z. Abidin, M.K.A. Mohamadiah, J. Hazard. Mater. 181, 755, (2010)