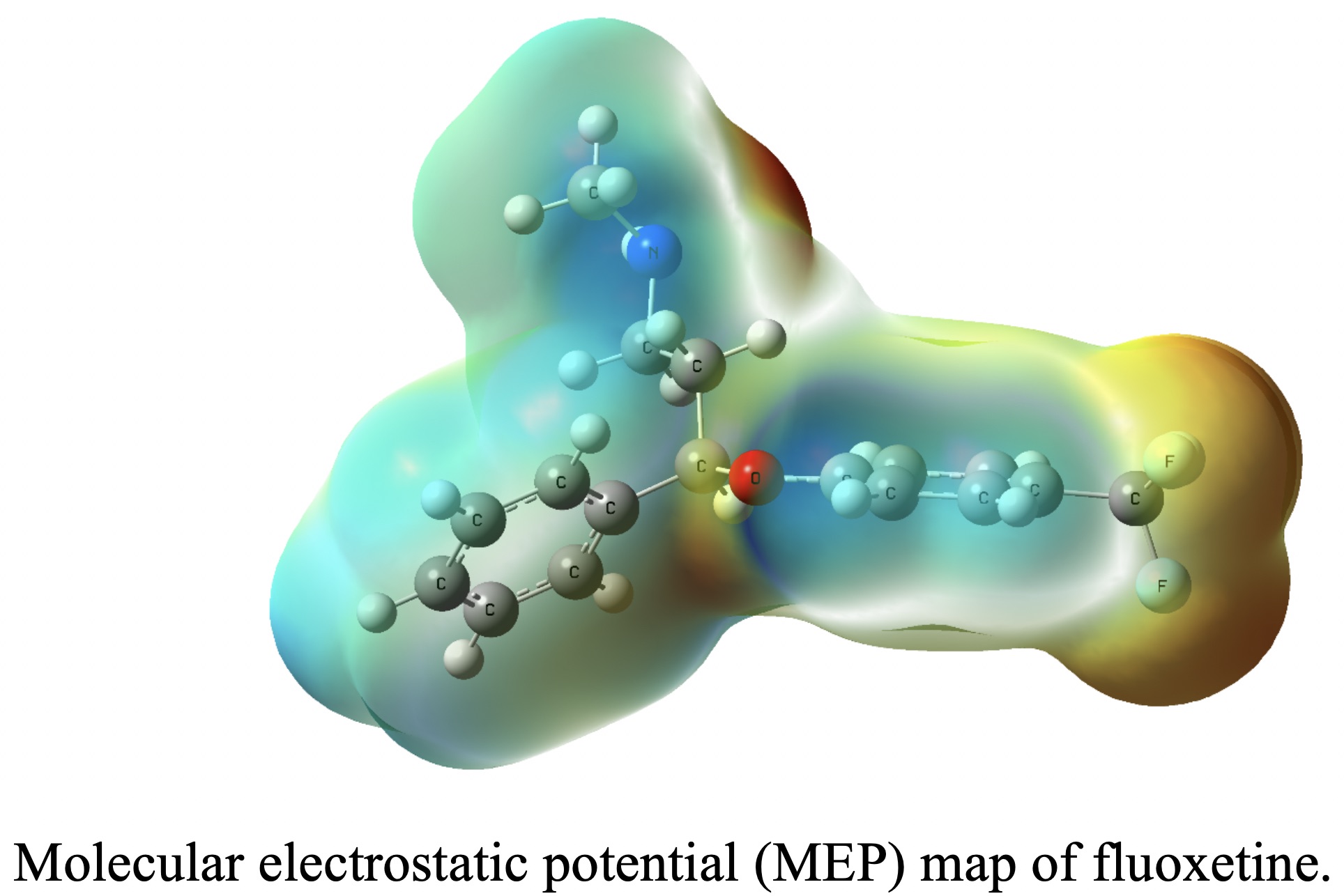
USING MOLECULAR ELECTROSTATIC POTENTIALS AND FRONTIER ORBITALS FOR THE SURFACE-ENHANCED RAMAN INTERPRETATION OF FLUOXETINE

- Raman
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Raman and surface-enhanced Raman (SERS) spectra of (N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy)propane-1-amine hydrochloride, fluoxetine, have been recorded. Density functional theory with the B3LYP functional was used for optimization of the ground state geometry, calculation of the Raman normal modes of this molecule and the modelling of the SERS effect. Calculated geometrical parameters of fluoxetine fit well with the experimental ones. Based on the recorded data, the DFT results and a normal coordinate analysis based on a scaled quantum mechanical (SQM) force field approach, a complete vibrational assignment of fluoxetine as well as its adsorption behavior on a silver surface (using SERS selection rules) is derived.
References
- Wong, D.T.; Bymaster, F.P.; Engleman, E.A. The First Selective Serotonin Uptake Inhibitor and an Antidepressant Drug: Twenty Years since Its First Publication. Life Science 1995, 57(5), 411-441.
- Indhumathi, D.; Grace, R. Design and optimization of orodissolving tablet of antidepressant drug by superdisintegrants addition method. Int. J. Pharm. Sci. Rev. Res. 2010, 2(2), 1-9.
- Persona, K.; Madej, K.; Knihnicki, P.; Piekoszewski, W. Analytical Methodologies for the Determination of Benzodiazepines in Biological Samples. J. Pharm. Biomed. Anal. 2015, 113, 239-264.
- Risley, D.S.; Bopp, R.J. in Analytical Profiles of Drugs Substances, Florey, K. Ed.; Academic Press: San Diego 1990; Vol. 19, pp. 193-219.
- Yellamula, N.R. ; Appapurapu, A.K.; Reddy, T.P.K.; Banji, D.; Appapurapu, A.K.; Deepthi, P.N. Effect of natural, synthetic and co-processed excipients on drug release of fluoxetine hydrochloride immediate release drug delivery system. Pharma Innovation, 2014, 3(8), 1-9.
- Garrido, E.M.; Garrido, J.; Calheiros, R.; Marques, M.P.M.; Borges, F. Fluoxetine and Norfluoxetine Revisited: New Insights into the Electrochemical and Spectroscopic Properties. J. Phys. Chem. A, 1994, 113(36), 9934–9944.
- Menaa, F.; Menaa B.; Sharts, O. Development of carbon-fluorine spectroscopy for pharmaceutical and biomedical applications. Faraday Discuss, 2011, 149, 269–278.
- King, F.W.; Van Duyne, R.P.; Schatz, G.C. Theory of Raman Scattering by molecules adsorbed on electrodes surface. J. Chem. Phys. 1978, 69(10), 4472–4481.
- Pinzaru, S.C.; Pavel, I.; Leopold, N.; Kiefer, W. Identification and characterization of pharmaceuticals using Raman and surface‐enhanced Raman scattering. J. Raman Spectrosc. 2004, 35, 338-346.
- Takenaka, M.; Hashimoto, Y.; Iwasa, T.; Taketsugu, T.; Seniutinas, G.; Balcytis, A.; Juodkazis, S.; Nishijima, Y. First Principles Calculations Toward Understanding SERS of 2,2‘-Bipyridyl Adsorbed on Au, Ag, and Au–Ag Nanoalloy. J. Comput. Chem. 2019, 40, 925–932.
- Luo, Z.; Loo, B. H.; Cao, X.; Peng, A.; Yao, J. Probing the Conformational Transition of 2,2’-Bipyridyl under External Field by Surface-Enhanced Raman Spectroscopy. J. Phys. Chem C 2012, 116, 2884-2890.
- Diaz F., G.; Finnerty, J. J.; Campos-Vallette, M.; Célis, F.; Aliaga, A. E.; Fredes, C.; Koch, R. Experimental and theoretical Raman and surface-enhanced Raman scattering study of cysteine. J. Raman Spec. 2009, 40, 632 – 638.
- Diaz F., G.; Golsio, I.; Aracena, A.; Celis, F.; Vera, L.; Koch, R.; Campos-Vallette, M. M. Theoretical Surface-Enhanced Raman Spectra study of substituted benzenes I. Density Functional Theoretical SERS modelling of benzene and benzonitrile. Spectrochimica Acta Part A 2008, 71, 1049 – 1055.
- Diaz F., G.; Golsio, I.; Aracena, A.; Celis, F.; Vera, L.; Koch, R.; Campos-Vallette, M. M. Theoretical Surface-Enhanced Raman Spectra study of substituted benzenes II. Density Functional Theoretical SERS modelling of o-, m-, and p-methoxybenzonitrile. Spectrochimica Acta Part A, 2008, 71, 1074 – 1079.
- Diaz F., G.; Célis, F.; Fredes, C.; Campos-Vallette, M.; Aliaga, A. E.; Koch, R. Surface-enhanced Raman scattering and density functional theory studies of bis(4-aminophenyl)sulfone. J. Raman Spec. 2010, 41, 160 – 166.
- Ejorh, Y. E.; Ilsley, W. H.; Ooi, B. G.. Elucidating the Chemisorption Phenomena in SERS Studies via Computational Modeling. Opt. Phot. J. 2018, 8, 212-234.
- Birke, R. L.; Lombardi, J. R. Simulation of SERS by a DFT study: a comparison of static and near-resonance Raman for 4-mercaptopyridine on small Ag clusters. J. Opt. 2015, 17, 114004.
- Zhao, L. L.; Jensen, L.; Schatz, G. C. Pyridine-Ag20 cluster: a model system for studying surface-enhanced Raman scattering. J. Am. Chem. Soc. 2006, 128, 2911–2919.
- Carrasco Flores, E. A.; Campos Vallette, M. M.; Clavijo C., R. E.; Leyton, P.; Díaz F., G.; Koch, R. SERS spectrum and DFT calculations of 6-nitrochrysene on silver islands. Vibrat. Spec. 2005, 37, 153 – 160.
- Hu, W.; Duan, S.; Luo, Y. Theoretical modeling of surface and tip-enhanced Raman spectroscopies. WIREs Comput Mol Sci 2017, 7:e1293.
- Muniz-Miranda, M.; Gellini, C.; Pagliai, M.; Innocenti, M.; Salvi, P. R.; Schettino, V. SERS and Computational Studies on MicroRNA Chains Adsorbed on Silver Surfaces. J. Phys. Chem. C 2010, 114, 13730-13735.
- Riccia, M.; Becuccia, M.; Castelluccia, E. M. Chemical enhancement in the SERS spectra of indigo: DFT calculation of the Raman spectra of indigo-Ag14 complexes. Vibrat. Spec. 2019, 100, 159-166.
- Pulay P.; Fogarasi G.; Pongor G.; Boggs J.E.; Vargha A. Combination of theoretical ab initio and experimental information to obtain reliable harmonic force constants. Scaled quantum mechanical (QM) force fields for glyoxal, acrolein, butadiene, formaldehyde, and ethylene. J. Am. Chem. Soc. 1983, 105(24), 7037-7047.
- Diaz Fleming, G.; Celis, F.; Aracena, A.; Campos-Vallette, M.; Aliaga, A. E.; Koch, R. Vibrational and scaled quantum chemical study of O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate, dimethoate. Spectrochimica Acta Part A 2012, 89, 222-230.
- Diaz Fleming, G.; Villagrán, J.; Koch, R. IR, Raman and SERS Spectral Analysis and DFT Calculations on the Herbicide O,S-Dimethyl phosphoramidothioate, Metamidophos. Spectrochimica Acta Part A 2013, 114, 120-128.
- Diaz Fleming, G.; Koch, R.; Muñoz Perez, J.; Llanos Cabrera, J. Raman and SERS Study of N-Acetyl-5-methoxytryptamine, melatonin - The influence of the different molecular fragments on the SERS effect. Vibrat. Spect. 2015, 80, 70-78.
- Campion, A., Kambhampati, P., Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27(4), 241-250.
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride.J. Phys. Chem. 2003, 107(24), 5723 – 5727.
- Cyrankiewicz, M.; Wybranowski, T.; Kruszewski, S. Study of SERS efficiency of metallic colloidal systems. J. Phys. Conf. Ser. 2007, 79, 012013.
- Vidhu, V.K.; Aromal, A.; Philip, D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta A 2011, 83(1), 392–397.
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomat. 2015, 2015, Article ID 720654.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H.P.; Izmaylov, A.F.; Bloino, J.; Zheng, G.; Sonnenberg, J.L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery Jr., J.A.; Peralta, J.E.; Ogliaro, F.; Bearpark, M.; Heyd, J.J.; Brothers, E.; Kudin, K.N.; Staroverov, V.N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J.M.; Klene, M.; Knox, J.E.; Cross, J.B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R.E.; Yazyev, O.; Austin, A.J.; Cammi, R.; Pomelli, C.; Ochterski, J.W.; Martin, R L.; Morokuma, K.; Zakrzewski, V.G.; Voth, G.A.; Salvador, P.; Dannenberg, J.J.; Dapprich, S.; Daniels, A.D.; Farkas, Ö.; Foresman, J.B.; Ortiz, J.V.; Cioslowski, J.; Fox, D.J. Gaussian 09, Rev. D01: Gaussian, Inc., Wallingford CT, 2009.
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98(7), 5648-5652.
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B: Condens. Matter 1988, 37, 785-789.
- Raghavachari, K.; Binkley, R.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. 20. Basis set for correlated wave-functions. J. Chem. Phys. 1980, 72, 650-654.
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z=11-18. J. Chem. Phys. 1980, 72, 5639-5648.
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.v.R. Efficient diffuse function-augmented basis-sets for anion calculations. 3. The 3-21+G basis set for 1st-row elements, Li-F. J. Comput. Chem. 1983, 4, 294-301.
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self‐consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80 (7) 3265-3269.
- Suh, I.K.; Ohta, H.; Waseda, Y. High-temperature thermal expansion of six metallic elements measured by dilatation method and X-ray diffraction. J. Mater. Sci. 1998, 23(2), 757-760.
- Andrae, D.; Häussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77(2), 123-141.
- Collier, W.C. QCPE Bull. 1996, 13, 16502-16513.
- Arky, R. Physicians’ Desk Reference, Medical Economics Data Production Company: Montvale, NJ, USA, 1994.
- Robertson, D.W.; Jones, N.D.; Swartzendruber, J.K.; Yang, K.S.; Wong, D.T. Molecular structure of fluoxetine hydrochloride, a highly selective serotonin-uptake inhibitor. J. Med. Chem. 1988, 31(1), 185-189.
- Zerbi, G. in Vibrational Spectroscopy-Modem Trends, Barnes, A.J.; Orville-Thomas, W.J., Eds.; Elsevier: New York, 1997.
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd Ed., John Wiley & Sons: New York/Brisbane/Weinheim/Singapore/Toronto, 2004.
- Vien, D.L.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, Academic Press: Boston, 1991.
- Varsanyi, G. Vibrational Spectra of Benzene Derivatives; Academic Press: New York, 1969.
- Stewart, J.E. Vibrational spectra of primary and secondary aliphatic amines. J. Chem. Phys. 1959, 30(5), 1259–1265.
- Shahidha, R.; Muthu, S.; Elamurugu Porchelvi, E.; Govindarajan, M. Normal Coordinate Analysis and Vibrational Spectroscopy (FT-IR and FT-Raman) Studies of 5-Methyl-N-[4-(Trifluoromethyl) Phenyl]-Isoxazole-4-Carboxamide Using Density Functional Method. Spectrochim. Acta A 2014, 132, 142–151.
- Kambhampati, P.; Child, C.M.; Foster, M.C.; Campion, A. On the chemical mechanism of surface enhanced Raman scattering: Experiment and theory. J. Chem. Phys. 1998, 108(12), 5013-5026.
- Lombardi, J.R.; Birke, R.L.; Lu, T.; Xu, J. Charge‐transfer theory of surface enhanced Raman spectroscopy: Herzberg–Teller contributions. J. Chem. Phys. 1986, 84(8), 4174-4180.
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57(3), 783-826.
- Creighton, J.A. Surface Raman electromagnetic enhancement factors for molecules at the surface of small isolated metal spheres: The determination of adsorbate orientation from SERS relative intensities. Surf. Sci. 1983, 124(1), 209-219.
- Gao, X.; Davies, J.P.; Weaver, M.J. Test of surface selection rules for surface-enhanced Raman scattering: the orientation of adsorbed benzene and monosubstituted benzenes on gold. J. Phys. Chem. 1990, 94(17), 6858-6864.
- Otto, A. Theory of First Layer and Single Molecule Surface Enhanced Raman Scattering (SERS) Phys. Status Solidi A 2001, 188(4), 1455-1470.
- Politzer, P.; Truhlar, D.G., Eds.; Chemical Application of Atomic and Molecular Electrostatic Potentials; Plenum: New York, 1981.
- Wang, Y.; Ji, W.; Sui, H.; Kitahama, Y.; Ruan, W.; Ozaki, Y.; Zhao, B. Exploring the Effect of Intermolecular H Bonding: A Study on Charge-Transfer Contribution to Surface-Enhanced Raman Scattering of p Mercaptobenzoic Acid. J. Phys. Chem. C 2014, 118(19), 10191−10197.
- Zhang, X.; Yu, Z.; Ji, W.; Sui, H.; Cong, Q.; Wang, X.; Zhao, B. Charge-Transfer Effect on Surface-Enhanced Raman Scattering (SERS) in an Ordered Ag NPs/4-Mercaptobenzoic Acid/TiO2 System. J. Phys. Chem. C 2015, 119(39), 22439−22444.
- Persson, B.N.J.; Ryberg, R. Vibrational interaction between molecules adsorbed on a metal surface: The dipole-dipole interaction. Phys. Rev. B 1981, 24(12), 6954–6970.
- Clark, R.J.H.; Dines, T.J. Resonance Raman Spectroscopy, and Its Application to Inorganic Chemistry. New Analytical Methods. Angew. Chem. Int. Ed. Engl. 1986, 25(2), 131-158.
- Persson, B.N.J. On the theory of surface-enhanced Raman scattering. Chem. Phys. Lett. 1981, 82(3), 561-565.
- Bjerneld, E.J.; Johansson, P.; Kaell, M. Single Molecule Vibrational Fine‐structure of Tyrosine Adsorbed on Ag Nano‐Crystals. Single Mol. 2000, 1, 239-248.
- Grabhorn, H.; Otto, A. What determines the selection rules of surface enhanced Raman spectroscopy? Vacuum 1990, 41, 473-475.
- Kittel, C. lntroduction to Solid State Physics. 5th Ed., John Wiley & Sons: New York, 1976.
- Glendening, E. D.; Reed, A. E.; Carpenter, J. E.; Weinhold, F. NBO Version 3.1.
- Reed, A. E. ; Curtiss, L. A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899-926.
- Lombardi, J. R.; Birke, R.L. A Unified Approach to Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2008, 112 (14), 5605− 5617.

