
- mass spectrometry,
- L-DOPA,
- Vicia faba,
- LC-MS/MS
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
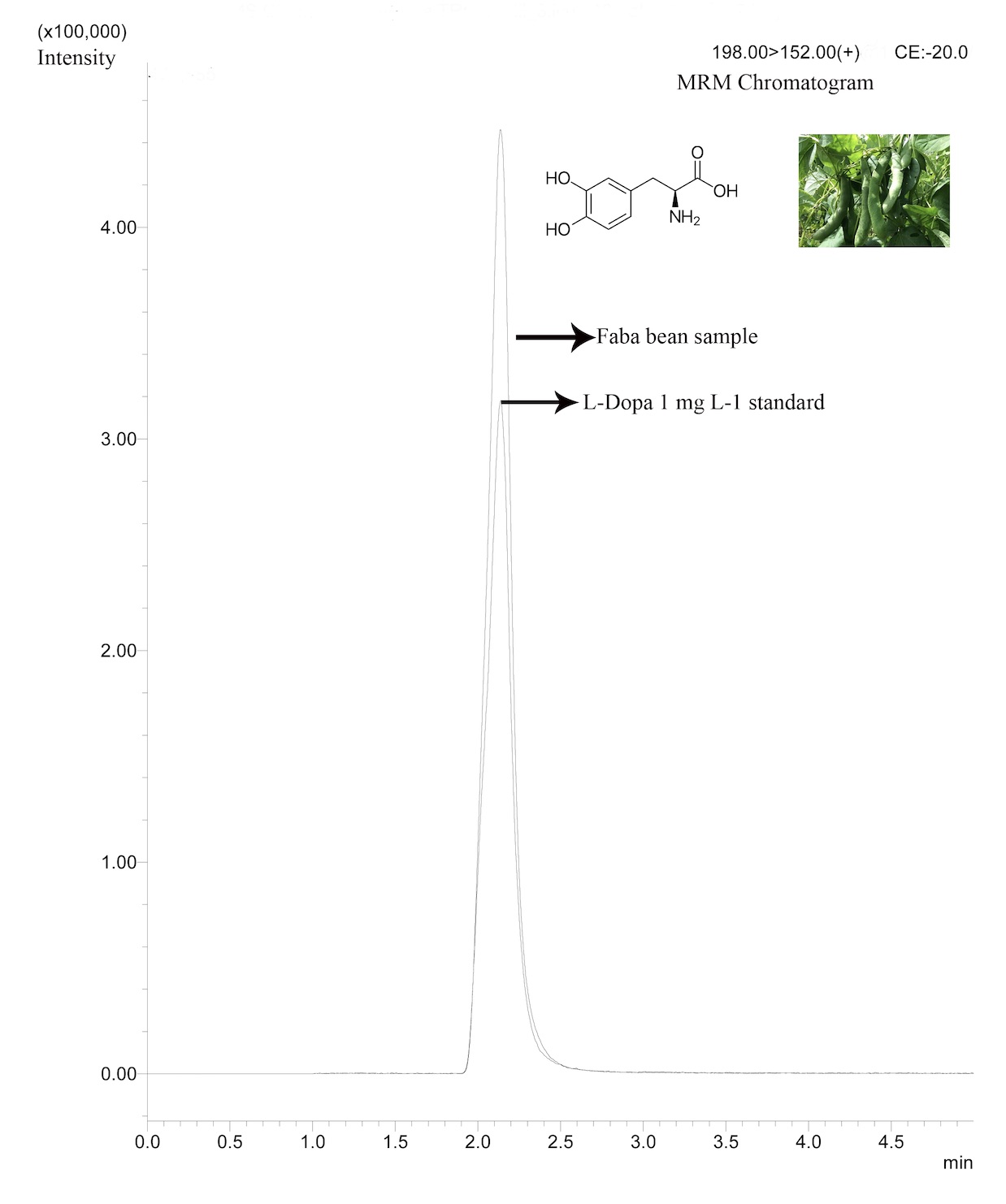
A rapid, sensitive, precise and accurate liquid chromatography tandem mass spectrometry (LC-MS/MS) method was developed for levo 3,4-dihydroxyphenylalanine (L-DOPA) determination in Vicia faba during different growth stages. The method applied a simple sample preparation step followed by a chromatographic separation on a Kinetex XB Core-Shell C18 (100 mm x 4.6 mm, 2.6 µm) column, using a mixture of ultrapure pure water (A) with 0.5 % (v/v) formic acid and methanol (B) as mobile phase. Analysis of L-DOPA was carried out by MS/MS applying a Multiple Reaction Monitoring (MRM) method using the transition m/z 198 → m/z 152. This LC-MS/MS method allowed a well-resolved detection of L-DOPA in ca. 2 min within 6 min run. Method was validated showing a linear range from 0.05 to 10 mg L-1 (R2 = 0.99); repeatability showed RSD value of 1.40%. Recoveries ranged from 94.14 to 116.62% with RSD values ≤ 5.66% and detection and quantification limits were 0.01 and 0.05 mg L-1, respectively. Applying this validated method, L-DOPA was determined in Vicia faba samples to determined its tissue distribution. As expected, a broad range of L-DOPA content finding values from 4.72 (in seeds) up to 133.60 mg g-1 (sprouts).
References
- S. Patil, O. Apine, S. Surwase, J. Jadhav, APD. 2, 7, (2013).
- F. Etemadi, M. Hashemi, R. Randhir, O. ZandVakili, A. Ebadi, J. Crop Prod. 6, 426, (2018).
- R. Randhir, K. Shetty, Process Biochem. 39, 1775, (2004).
- C.M. Gautam M, Azmi W, Ann Phytomed. 1, 1, (2012).
- S. Nidhi, Ann. Plant Sci. 4, 1109, (2015).
- S. Inamdar, S. Joshi, J. Jadhav, V. Bapat, Nat. prod. bioprospect. 2, 16, (2012).
- L. Raguthu, S. Varanese, L. Flancbaum, E. Tayler, A. Di Rocco, Eur J Neurol. 16, 171, (2009).
- M. Mehran S M, G. B, J Clin Diagn Res : JCDR. 7, 1004, (2013).
- A.P. Raina, R. Khatri, Indian J. Pharm. Sci. 73, 459, (2011).
- C. Burbano, C. Cuadrado, M. Muzquiz, J.I. Cubero, Plant Foods Hum Nutr. 47, 265, (1995).
- C. Oviedo, M. Elso-Freudenberg, M. Aranda, Appl. Sci. 8, 2431, (2018).
- K. Igarashi, K. Hotta, F. Kasuya, K. Abe, S. Sakoda, J. chromatogr. B. 792, 55, (2003).
- W. Li, D.T. Rossi, S.T. Fountain, J Pharm Biomed Anal. 24, 325, (2000).
- International Conference on Harmonisation (ICH), 2005.
- R. Singh, P. Saini, S. Mathur, G. Singh, S. Kumar, Int. J. Green Pharm. 4, 156, (2010).
- S. Raghavendra, C.K. Ramesh, V. Kumar, M.H. Moinuddin Khan, Front Life Sci 5, 127, (2011).
- C.A. Schenck, H.A. Maeda, Phytochemistry 149, 82, (2018).
- C. Goyoaga, C. Burbano, C. Cuadrado, A. Varela, E. Guillamón, M.M. Pedrosa, M. Muzquiz, Eur Food Res Technol. 227, 1537, (2008).


