ELECTROCHEMICAL DETERMINATION OF N,N-DIMETHYLTRYPTAMINE IN WATER BASED ON TETRARUTHENATED PORPHYRINS AND IONIC LIQUID MODIFIED ELECTRODES

- Tetraruthenated porphyrins,
- Modified electrodes,
- Ionic liquids,
- DMT
Copyright (c) 2020 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
N,N-dimethyltryptamine (DMT) is a short-acting psychotropic agent when administered parenterally and is not active orally due to its rapid degradation by monoamine oxidase enzyme type A. There is growing interest in the therapeutic potential of DMT due to recent clinical data that has shown that produces antidepressant effects in humans. It has also been found that psychedelics that possess the central structure of DMT may have value as medications and cannot simply be labeled as drugs with the potential for abuse.
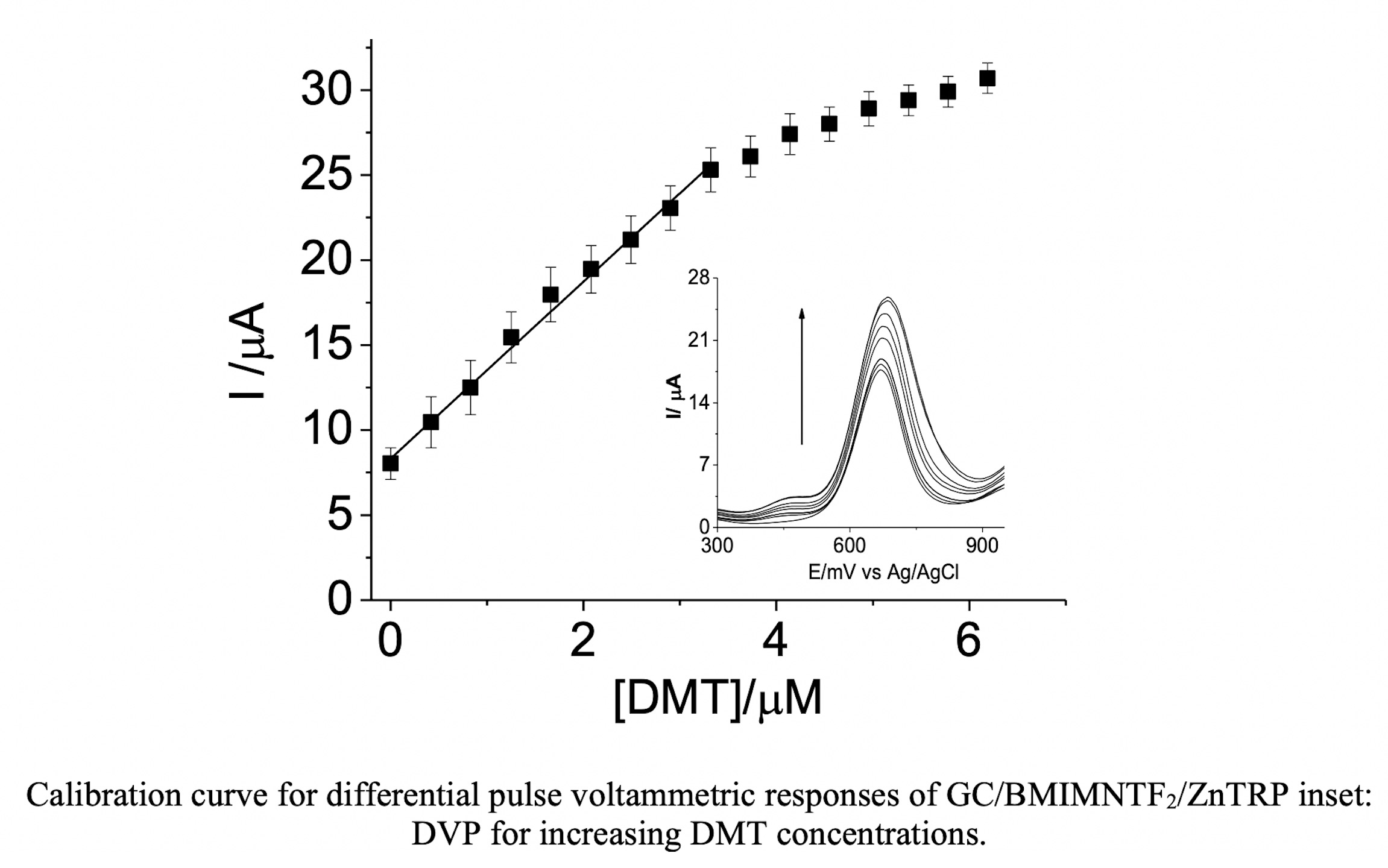
The incorporation of ionic liquid and tetraruthenated zinc porphyrin to glassy carbon electrode enhanced the voltammetric determination of DMT compared to the bare glassy carbon electrode. These modified electrodes showed good performance for DMT oxidation. These results translate into a low detection limit (1.75 µM), short response time, satisfactory linear concentration range, very good stability and reproducibility. Thus, his methodology can be a feasible and inexpensive way to detect DMT in water and presents potential to be applied in the detection of DMT in urine.
References
- S. Navickiene, L.F.S. Santos, M.C. Santos, A. Gaujacb, J. Braz. Chem. Soc, 30 (2019) 180-187.
- L.E. Dunlap, D.E. Olson, ACS Omega, 3 (2018) 4968-4973.
- http://www.fiscaliadechile.cl/Fiscalia/sala_prensa/noticias_regional_det.do?id=16131. Last visited on October 30, 2019
- https://www.latercera.com/nacional/noticia/carabineros-desbarata-primer-laboratorio-chile-la-droga-los-dioses/673756/. Last visited on October 30, 2019
- S. D. Brandt, C.P.B. Martins, TrAC, 29 (2010) 858-869.
- M. Yritia, J. Riba, J. Ortuño, A. Ramirez, A. Castillo, Y. Alfaro, R. De La Torre, M.J. Barbanoj, J. Chromatogr. B., 779 (2002) 271-281.
- A. Gaujac, A. Aquino, S. Navickiene, J. Bittencourtde de Andrade, J. Chromatogr. B., 881-882 (2012) 107-110.
- A. Gaujac, N. Dempster, S. Navickiene, S.D. Brand, J. Bittencourtde de Andrade, Talanta, 106 (2013) 394-398.
- P. Rodriguez de Morais, R. Llanaro, Forensic Toxicol., 36 (2018) 212-221.
- A. Wohlfarth, W. Weinmann, S. Dresen, Anal Bioanal Chem, 396 (2010) 2403- 2414.
- L. Ambach, A. Hernández Redondo, S. König, W. Weinmann, Drug Test Anal., 6 (2014) 367-375.
- D. Zhang, Y. Wang, W. Geng, H. Liu, Sensor Actuator B-Chem. 285 (2019) 546-552.
- S. Navickiene, L.F.S. Santos, M.C. Santos, A. Gaujac, J. Braz. Chem. Soc. 30 (2019) 180-187.
- J. Wang, Analytical Electrochemistry, Willey-VCH, New York 2001.
- N. Alonso-Vante, Electroquimica y electrocatalisis, 1st ed.virtual e-libro.net 2003.
- K. Calfumán, D. Quezada, M. Isaacs, S. Bollo, Electroanal., 27 (2015) 2778-2784.
- K. Calfumán, J. Honores, M. Isaacs, D. Quezada, J. Valdebenito, M. Urzua, Electroanal., 31 (2019) 671-677.
- F. Crespilho, M. Ghica, M. Florescu, F. Nart, O. Oliveira Jr, C. Brett, Electrochem.
- Commun. 8 (2006) 1665-1670.
- M. Aguirre, E. Trollund, P. Ardiles, S. Biaggio, R. Rocha- Filho, Polyhedron, 19 (2000) 2303-2312.
- J. Zagal, Coord. Chem. Rev. 119 (1992) 89-136.
- Y. Guan, L. Liu, C. Chen, X. Kang, Q. Xie, Talanta, 160 (2016) 125-132.
- M. S. M. Quintino, K. Araki, H. E. Toma, L. Angnes, Talanta, 68 (2006) 1281–1286.
- M. S. M. Quintino, H. Winnischofer, K. Araki, H. E. Toma, L. Angnes, Analyst, 130 (2005) 221–226.
- A. Prodi, M. T. Indelli, C. J. Kleverlaan, E. Alessio, F. Scandola, A. Prodi, M. T. Indelli, C. J. Kleverlaan, E. Alessio, F. Scandola, Coord. Chem. Rev., 229 (2002) 51–58.
- K. Calfumán, M. J. Aguirre, D. Villagra, C. Yañez, C. Arévalo, B. Matsuhiro, M. Isaacs, J. Solid State Electrochem., 14 (2010) 1065–1072.
- K. Calfumán, M. García, M. J. Aguirre, B. Matsuhiro, L. Mendoza, M. Isaacs, Electroanal., 22 (2010) 338–344.
- P. Dreyse, D. Quezada, J. Honores, M. J. Aguirre, L. Mendoza, B. Matsuhiro, D. Villagra, M. Isaacs, Electroanal., 24 (2012) 1709–1718.
- F. C. Anson, C. Shi, B. Steiger, Acc. Chem. Res., 30 (1997) 437–444.
- P. Dreyse, J. Honores, D. Quezada, M. Isaacs, Chem. Sus. Chem. 8 (2015) 3897-3904.
- J. Honores, D. Quezada, M. García, K. Calfumán, J.P. Muena, M.J. Aguirre, M.C. Arévalo, M. Isaacs, Green Chem., 19 (2017) 1155-1162.
- K. Calfumán, P. Dreyse, C. Garcia, M. J. Aguirre, T. Beltran, E. Guillamón, I. Sorribes, C. Vicent, R. Llusar, M. Isaacs, Macromol. Symp. 304 (2011) 93–100.
- T. Welton, Chem. Rev., 99 (1999) 2071-2084.
- P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. Engl., 39 (2000) 3772-3789.
- M. Opallo, A. Lesniewski, J. of Electroanal. Chem., 656 (2011) 2-16.
- A. Gaujac, S. Teixeira Martínez, A. Araújo Gomes, S. Jose de Andrade, A. da Cunha Pinto, J. Mauricio David, S. Navickiene, J. Bittencourt de Andrade, Microchem. J., 109 (2013) 78–83.
- J. Schripsema, D. Dagnino, G. Gossman, Alcalóides Indólicos, in: C.M.O. Simões, E.P.
- Schenkel, G. Gosmann, J.C.P. Mello, L.A. Mentz, P.R. Petrovick (Eds.), Farmacognosia:
- da Planta ao Medicamento, Editora UFRGS, Porto Alegre, Editora UFSC, Florianópolis,
- , pp. 819–846.
- K. Araki, H. Toma, J. Photochem. Photobiol. 83 (1994) 245-250.
- K. Araki, H. toma, Coord. Chem. Rev. 196 (2000) 307-329.
- K. Calfuman, M.J.Aguirre, D.Villagra, C.Yañez, C.Arévalo, B. Matsuhiro, M. Isaacs, J. Solid State Electrochem. 14 (2010) 1065–1072.
- K. Calfumán, D. Quezada, M. Isaacs, S. Bollo, Electroanal., 27 (2015) 2778–2784.
- M.M. Gomes, J.B. Coimbra, R.O. Clara, F.A. Dörr, A.C.R. Moreno, J.R. Chagas, S. Tufik, E. Pinto Jr, L.H. Catalani, A. Campa, Biochem. Pharmacol., 88 (2014) 393-401.
- K. Calfumán, J. Honores, M. Isaacs, D. Quezada, J. Valdebenito, M. Urzua, Electroanal., 31 (2019) 671-677.
- A. Gaujac, N. Dempster, S. Navickiene, S.D. Brandt, J. Bittencourt de Andrade, Talanta, 106 (2013) 394-398.
- K. Björnstad, O. Beck, A. Helander, J. Chromatogr. B, 877 (2009) 1162-1168.
- T. Forsström, J. Tuominen, J. Kärkkäinen, Scand J Clin Lab Invest, 61 (2001) 547-556.
- M.R. Meyer, A. Caspar, S.D. Brandt, H.H. Maurer, Anal. Bioanal. Chem, 406 (2014) 225-237.
- S.P. Vorce, J.H. Sklerov, J. Anal. Toxicol., 28 (2004) 407-410.
- J. Riba, E.H. Mcllhenny, M. Valle, J.C- Bouso, S.A. Barker, Drug Test. Anal., 4 (2012) 610-616.

