Vol 64 No 3 (2019): Journal of the Chilean Chemical Society
Original Research Papers
ANALYTICAL APPROACHES IN UNVEILING THE COMPATIBILITY OF COLLAGENASE WITH ADDITIVES IN VIEW OF ITS COLLOIDAL DELIVERY
Published
October 30, 2019
Keywords
- Collagenase,
- FTIR-ATR,
- SDS-PAGE,
- Circular Dichroism
How to Cite
Vijay, L. A., & Kannan, K. (2019). ANALYTICAL APPROACHES IN UNVEILING THE COMPATIBILITY OF COLLAGENASE WITH ADDITIVES IN VIEW OF ITS COLLOIDAL DELIVERY. Journal of the Chilean Chemical Society, 64(3), 4558-4564. Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/1320
Abstract
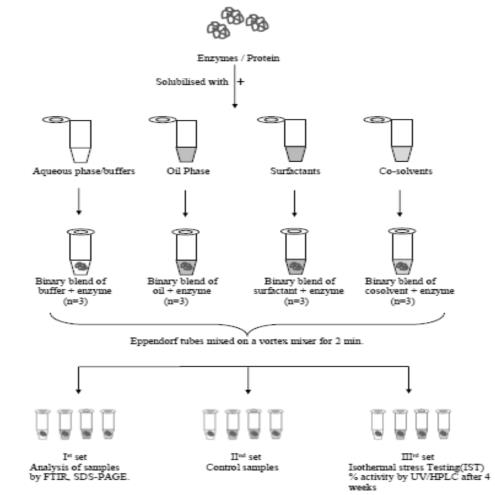
The core objective of present work was to investigate the chemical interactions of selected additives with therapeutic enzyme Collagenase (COLG) and determination of its compatibility for futuristic colloidal formulation. Initially, solubility of COLG with additives was determined and emulsification efficiency of surfactants was examined. FTIR spectra of COLG with its binary blends were compared. Non-denaturing SDS-PAGE and Circular Dichroism (CD) used to investigate primary and secondary structure of enzyme in binary mixture. Finally, the control and accelerated studies was performed by spectroscopy.
References
1. W. Wang, Advanced protein formulations, Protein sci. 24, 7, (2015).
2. L. Anil, K. Kannan, J. Pharm. Sci. Res. 10, 11, (2018).
3. N. Nezafat, M. Negahdaripour, A. Gholami, Y. Ghasemi, Trends Pharm. Sci. 1, 4, (2015).
4. M. H. E. Nagar, M. Mahdy, M. Selem, G. E. Maghraby, J. App. Pharm. Sci. 6, 3, (2016).
5. H. Y. Karasulu, B. Karabulut, G. Kantarci, I. Ozgüney, C. Sezgin, U. A. Sanli, E. Göker, Drug Deliv. 11, 6, (2004).
6. K. Krishnamoorthy, A. V. Landge, Braz. Arch. Biol. Technol. 61, (2018).
7. A. Shalviri, A.C. Sharma, D. Patel, A. Sayani, J. Pharm. Pharm. Sci. 14, 3 (2011).
8. P. Bummer, in Protein formulation and delivery. E. McNally, J. Hastedt eds, CRC press, Florida, 2008; pp.7-45.
9. A. A. Date, M. Nagarsenker, Int. J. Pharm. 329, 1, (2007).
10. A. Barth, Biochim. Biophys. Acta. 1767, 9, (2007).
11. G. M. Kirsten, L. M. Blancas-Mejia, B. Weber, J. Buchnar, Amyloid. 24, 1, (2007).
12. N. R. Pani, L. Nath, S. Acharya, Acta Pharm. 61, 2 (2011).
13. K. Pramod, C. V. Suneesh, S. Shanavas, J. Anal. Sci. Tech. 6, 34, (2015).
14. A. M. Cernada, J. C. Fernandez, L. P. Fernandez, L. R. Calvo, D. Rojas, G. Figueredo, A. Rodríguez, C. Fernández, T. Sosa, G. Amaro, Bioprocess Int. 15, 2, (2016).
15. US-FDA: Stability testing of new enzyme substances and products. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Q1A (R2), current step 4 version, (2003).
16. H. Anushree, and K. Ajay, Transdermal Delivery of Peptides and Proteins, in Peptide and protein delivery, W. Chrisvander eds. Elsevier, London, 2011; pp. 69-83.
17. J. Angelo, S. Hempstead, H. Edwin, United States patent no. 3705083 (1972).
18. M. Zdravka, S. Dubravka, S. Georg, K. Martin, J. Addit. Food Chem. 5, 2, (2014).
19. U. Laemmli, Nature. 227, 5259, (1970).
20. D. R. Perinelli, M. Cespi, S. Pucciarelli, S. Vincenzetti, L. Casettari, J. Lam, S. Logrippo, E. Canala, M. Soliman, Curr. Pharm. Biotechnol. 18, 5, (2017).
21. S. T. L. Philominathan, O. Matsushita, R. Gensure, J. Sakon, FEBS J. 276, 13, (2009).
22. N. Patil, P. Devarajan, Drug Deliv. Transl. Res. 4, 5, (2014).
23. H. Hossam, Indian J. Biotechnol. 7, 3, (2008).
24. S. M. Daboor, S. M. Budge, A. E. Ghaly, S. L. Brooks, D. Deepika, Am. J. Biochem. Biotechnol. 6, 4, (2010).
25. Y. S. Elnaggar, M. A. El-Massik, O. Y. Abdallah, Int. J. Pharm. 380, 1, (2009).
26. L. Wang, J. Dong, J. Chen, J. Eastoe, X. Li, J Colloid and Interface Sci. 330, 2, (2008).
27. D. A. Farghaly, A. A. Aboelwafa, M. Y. Hamza, M. I. Mohamed, J. Liposome Res. 28, 2, (2017).
28. J. Yang, G. Sule-Suso, D. Sockalingum, Proc. SPIE. 6859, (2008).
29. M. Magdy, A. Muharram, Saudi Pharm. J. 25, 3, (2017).
30. N. J. Greenfield, Nat. Protoc. 1, 6, (2006).
31. A. Micsonai, F. Wien, L. Kernya, Y. Lee, Y. Goto, M. Réfrégiers, J. Kardos, Proc. Natl. Acad. Sci. 112, 24, (2015).
32. W. Galbraith, European patent no. EP0039726A1 (1981).
2. L. Anil, K. Kannan, J. Pharm. Sci. Res. 10, 11, (2018).
3. N. Nezafat, M. Negahdaripour, A. Gholami, Y. Ghasemi, Trends Pharm. Sci. 1, 4, (2015).
4. M. H. E. Nagar, M. Mahdy, M. Selem, G. E. Maghraby, J. App. Pharm. Sci. 6, 3, (2016).
5. H. Y. Karasulu, B. Karabulut, G. Kantarci, I. Ozgüney, C. Sezgin, U. A. Sanli, E. Göker, Drug Deliv. 11, 6, (2004).
6. K. Krishnamoorthy, A. V. Landge, Braz. Arch. Biol. Technol. 61, (2018).
7. A. Shalviri, A.C. Sharma, D. Patel, A. Sayani, J. Pharm. Pharm. Sci. 14, 3 (2011).
8. P. Bummer, in Protein formulation and delivery. E. McNally, J. Hastedt eds, CRC press, Florida, 2008; pp.7-45.
9. A. A. Date, M. Nagarsenker, Int. J. Pharm. 329, 1, (2007).
10. A. Barth, Biochim. Biophys. Acta. 1767, 9, (2007).
11. G. M. Kirsten, L. M. Blancas-Mejia, B. Weber, J. Buchnar, Amyloid. 24, 1, (2007).
12. N. R. Pani, L. Nath, S. Acharya, Acta Pharm. 61, 2 (2011).
13. K. Pramod, C. V. Suneesh, S. Shanavas, J. Anal. Sci. Tech. 6, 34, (2015).
14. A. M. Cernada, J. C. Fernandez, L. P. Fernandez, L. R. Calvo, D. Rojas, G. Figueredo, A. Rodríguez, C. Fernández, T. Sosa, G. Amaro, Bioprocess Int. 15, 2, (2016).
15. US-FDA: Stability testing of new enzyme substances and products. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Q1A (R2), current step 4 version, (2003).
16. H. Anushree, and K. Ajay, Transdermal Delivery of Peptides and Proteins, in Peptide and protein delivery, W. Chrisvander eds. Elsevier, London, 2011; pp. 69-83.
17. J. Angelo, S. Hempstead, H. Edwin, United States patent no. 3705083 (1972).
18. M. Zdravka, S. Dubravka, S. Georg, K. Martin, J. Addit. Food Chem. 5, 2, (2014).
19. U. Laemmli, Nature. 227, 5259, (1970).
20. D. R. Perinelli, M. Cespi, S. Pucciarelli, S. Vincenzetti, L. Casettari, J. Lam, S. Logrippo, E. Canala, M. Soliman, Curr. Pharm. Biotechnol. 18, 5, (2017).
21. S. T. L. Philominathan, O. Matsushita, R. Gensure, J. Sakon, FEBS J. 276, 13, (2009).
22. N. Patil, P. Devarajan, Drug Deliv. Transl. Res. 4, 5, (2014).
23. H. Hossam, Indian J. Biotechnol. 7, 3, (2008).
24. S. M. Daboor, S. M. Budge, A. E. Ghaly, S. L. Brooks, D. Deepika, Am. J. Biochem. Biotechnol. 6, 4, (2010).
25. Y. S. Elnaggar, M. A. El-Massik, O. Y. Abdallah, Int. J. Pharm. 380, 1, (2009).
26. L. Wang, J. Dong, J. Chen, J. Eastoe, X. Li, J Colloid and Interface Sci. 330, 2, (2008).
27. D. A. Farghaly, A. A. Aboelwafa, M. Y. Hamza, M. I. Mohamed, J. Liposome Res. 28, 2, (2017).
28. J. Yang, G. Sule-Suso, D. Sockalingum, Proc. SPIE. 6859, (2008).
29. M. Magdy, A. Muharram, Saudi Pharm. J. 25, 3, (2017).
30. N. J. Greenfield, Nat. Protoc. 1, 6, (2006).
31. A. Micsonai, F. Wien, L. Kernya, Y. Lee, Y. Goto, M. Réfrégiers, J. Kardos, Proc. Natl. Acad. Sci. 112, 24, (2015).
32. W. Galbraith, European patent no. EP0039726A1 (1981).


