ANTIMICROBIAL ACTIVITY AND IN SILICO STUDY OF METHYLIMIDAZOLIUM-FURANCHALCONE HYBRIDS AND 1-ALKYL-3-METHYLIMIDAZOLIUM SALTS
- Antibacterial,
- antifungal,
- ionic liquid,
- in silico study,
- hybrid molecules
Abstract
A set of methylimidazolium-furanchalcone hybrids and 1-alkyl-3-methylimidazolium salts were tested in order to find their antimicrobial activity against five gram
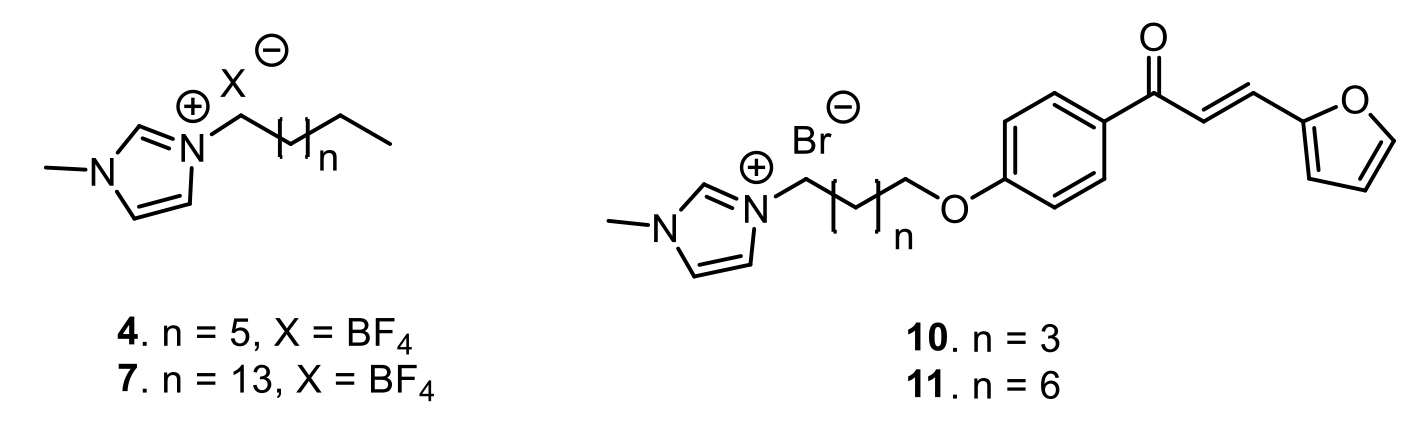
positive bacteria, two gram negative bacteria and three fungi. In the series of 1-alkyl-3-methylimidazolium salts, compounds 4 and 7 were the most active compounds
against S. mutans and F. oxysporum, respectively (MIC value of 3.0 and 6.7 mM, respectively). In addition, among the hybrid molecules, compound 10 exhibited the
highest activity against F. solani (MIC50 = 14.3 mM) followed by hybrid 11 (MIC50 = 14.5 mM), which was also the most active against S. aureus, S. mutans (MIC50
= 14.6, 18.7 mM, respectively). The activity of these hybrids was even better with regard to the lead compounds (furanchalcone, methylimidazole or the mixture). In
the structure-activity relationship there was observed higher bioactivity when the alkyl linker had eigth carbon atoms. This was then supported by the in silico study
which displayed a strong relationship between the length of the alkyl chain on N and the biological activity. The results highlight the importance of these derivatives
(1-alkyl-3-methylimidazolium salts) and hybrids (methylimidazolium-furanchalcone) as potential antimicrobial agents.
References
2. S.J. Howard, M. Catchpole, J. Watson, S.C. Davies, Lancet Infect. Dis., 13, 1001, (2013).
3. R. Laxminarayan, A. Duse, C. Wattal, A.K. Zaidi, H.F. Wertheim, N. Sumpradit, E. Vlieghe, G.L.Hara, I.M. Gould, H. Goossens, C. Greko, A.D. So, M. Bigdeli, G. Tomson, W. Woodhouse, E. Ombaka, A.Q. Peralta, F.N. Qamar, F. Mir, S. Kariuki, Z.A. Bhutta, A. Coates, R. Bergstrom, G.D.Wright, E.D. Brown, O. Cars, Lancet Infect. Dis. 3, 1057, (2013).
4. H.W. Boucher, G.H. Talbot, J.S. Bradley, J.E. Edwards, D. Gilberto, L.B. Rice, M. Scheld, B. Spellberg, J. Bartlett, Clin. Infect. Dis. 48, 1, (2009).
5. R.D. Cannon, E. Lamping, A.R. Holmes, K. Niimi, P.V. Baret, M.V. Keniya, K. Tanabe, M. Niimi, A. Goffeau, B.C. Monk, Clin. Microbiol. Rev. 22, 291, (2009).
6. P.G. Pappas, C.A. Kauffman, D. Andes, D.K. Benjamin Jr, T.F. Calandra, J.E. Edwards Jr, S.G. Filler, J.F. Fisher, B.J. Kullberg, L.O. Zeichner, C. Reboli, J.H. Rex, T.J. Walsh, J.D. Sobe. Clin. Infect. Dis. 48, 503 (2009).
7. J.M. Achkar, B.C. Fries. Clin. Microbiol. Rev. 23, 253 (2010).
8. M.K. Kathiravana, A.B. Salake, A.S. Chothe, P.B. Dudhea, R.P. Watodea, M.S. Muktaa, S. Gadhwea. Bioorg. Med. Chem. 20, 5678 (2012).
9. L. De Luca. Curr Med Chem 13, 1 (2006).
10. L. Zhang, X.M. Peng, G.L.V. Damu, R.X. Geng, C.H. Zhou. Med. Res. Rev. 34, 340 (2014).
11. D. Zampieri, M.G. Mamolo, L. Vio, E. Banfi, G. Scialino, M. Fermeglia, M. Ferrone, S. Pricl. Bioorg. Med. Chem. 15, 7444 (2007).
12. J. Gising, M.T. Nilsson, L.R. Odell, S. Yahiaoui, M. Lindh, H. Iyer, A.M. Sinha, B.R. Srinivasa, M. Larhed, S.L. Mowbray, A.J. Karlén. Med. Chem. 55, 2894 (2012).
13. A. Puratchikodya, M. Doble, Bioorg. Med. Chem. 15, 1083 (2007).
14. R. Selig, M. Goettert, V. Schattel, D. Schollmeyer, W. Albrecht, S. Laufer, J. Med. Chem. 55, 8429 (2012).
15. J. Pandey, V.K. Tiwari, S.S. Varma, V. Chaturvedi, S. Bhatnagar, S. Sinha, A.N. Gaikwad, R.P. Tripathi. Eur. J. Med. Chem. 44, 3350 (2009).
16. G. La Regina, F.D. D’Auria, A. Tafi, F. Piscitelli, S. Olla, F. Caporuscio, L. Nencioni, R. Cirilli, F. La Torre, N.R. De Melo, S.L. Kelly, D.C. Lamb, M. Artico, M. Botta, A.T. Palamara, R. Silvestri, J. Med. Chem. 51, 3841 (2008).
17. R.A. Al-Qawasmeh, M. Huesca, V. Nedunuri, R. Peralta, J. Wright, Y. Lee, Y. Young, Bioorg. Med. Chem. Lett. 20, 3518 (2010).
18. A. Cornellas, L. Perez, F. Comelles, I. Ribosa, A. Manresa, M.T. Garcia, J. Colloid Interface Sci. 355, 164 (2011).
19. P. Borowiecki, M. Milner-Krawczyk, D. Brzezinska, M. Wielechowska, J. Plenkiewicz, Eur. J. Org. Chem. 4, 712 (2013).
20. P. Mester, M. Wagner, P. Rossmanith. Ecotoxicol. Environ. Saf. 111, 96 (2015).
21. J. Yu, S. Zhang, Y. Dai, X. Lu, Q. Lei, W. Fang, J. Hazard Mater 307, 73 (2016).
22. J. Pernak, K. Sobaszkiewicz, I. Mirska, Green Chem. 5, 52 (2003).
23. D. Demberelnyamba, K.S. Kim, S.J. Choi, S.Y. Park, H. Lee, C.J. Kim, I.D. Yoo, Bioorg. Med. Chem. 12, 853 (2004).
24. A. Romero, A. Santos, J. Tojo, A. Rodriguez, J, Hazard Mater., J. Hazard Mater. 151, 268 (2008).
25. M.T. Garcia, N. Gathergood, P.J. Scammells, Green Chem. 7, 9 (2005).
26. J. Luczak, C. Jungnickel, I. Lacka, S. Stolte, J. Hupka. Green Chem. 12, 593 (2010).
27. a) A. Budhiraja, K. Kadian, M. Kaur, V. Aggarwal, A. Garg, S. Sapra, K. Nepali, O.P. Suri, K.L. Dhar, Med. Chem. Res. 21, 2133 (2012) b) S. Kantevari, D. Addla, P.K. Bagul, B. Sridhar, S.K. Banerjee. Bioorg. Med. Chem. 19, 4772 (2011) c) S.U.F. Rizvi, H.L. Siddiqui, M. Johns, M. Detorio, R.F. Schinazi, Med. Chem. Res. 21, 3741 (2012) d) D.A. Israf, T.A. Khaizurin A. Syahida, N.H. Lajis, S. Khozirah. Mol. Immunol. 44, 673 (2007) e) N. Aoki, M. Muko, E. Ohta, S. Ohta. J. Nat. Prod. 71, 1308 (2008) f) V. Tomar, G. Bhattacharjee, Kamaluddin, S. Rajakumar, K. Srivastava, S.K. Puri, Eur. J. Med. Chem. 45, 745 (2010) g) M. Chen, S.B. Christensen, J. Blom, E. Lemmich, L. Nadelmann, K. Fich, T.G. Theander, A. Kharazmi. Antimicrob. Agents Chemother. 37, 2550 (1993) h) M.I. Abdullah, A. Mahmood, M. Madni, S. Masood, M. Kashif, Bioorg. Chem. 54, 31 (2014) g) K.V. Lahtchev, D.I. Batovska, S.P. Parushev, V.M. Ubiyvovk, A.A. Sibirny, Eur. J. Med. Chem. 43, 2220 (2008).
28. M.A.Munawar, M. Azad, H.L. Siddiqui, J. Chin. Soc. 55, 394 (2008).
29. M. Azad, M.A. Munawar, H.L. Siddiqui, J. Appl. Sci. 7, 2485 (2007).
30. R. Kalirazan; S.U. Sivakumar, S. Julie, B. Gowramma, B. Suresh, Int. J. Chemtech. Res. 1, 27 (2009).
31. S.N. Lopez, V. Marıa Castelli, S.A. Zacchino. Bioorg. Med. Chem. 9, 1999 (2001).
32. C.T. Keith, A. Borisy, B.R. Stockwell. Nat. Rev. Drug Discov. 4, 71 (2005).
33. B. Meunier, Acc. Chem. Res. 41, 69 (2008).
34. I. Opsenica, D. Opsenica, C.A. Lanteri, L. Anova, W.K. Milhous, K.S. Smith, B.A. Solaja, J. Med.Chem. 51, 6216 (2008).
35. B.L. Roth, D.J. Sheffler, W.K. Kroeze, Nat. Rev. Drug Discov. 3, 353 (2004).
36. J.J. Walsh, D. Coughlan, N. Heneghan, C. Gaynor, A. Bell, Bioorg. Med. Chem. Lett. 17, 3599. (2007).
37. P. Araque, C. Peláez, Revista Vitae 17, 83 (2010).
38. A.W. Bauer, W.M.M. Kirby, J.C. Sherris, M. Turck. Am. J. Clin. Pathol. 45, 493 (1966).
39. E.P. Abraham, A.D. Gardner, E.B. Chain, N.G. Heatley, C.M. Fletcher, M.A. Jennings, H.W. Florey, Lancet ii, 177 (1941).
40. I. Hossain, M.E. Harbawi, Y.A. Noaman, M.A.B. Bustam, N.B.M. Alitheen, N.A. Affandi, G. Hefter, C.Y. Yin. Chemosphere 84, 101 (2011).
41. K. Silva, S. Rodrigues, L. Filho, A. Lima, Appl. Biochem. Biotechnol. 152, 316 (2009).
42. Finney, D. J., 3rd Ed. (1971). Probit Analysis. Cambridge, England, Cambridge University Press.
43. Y. Zhao, M.H. Abraham, J. Lee, A. Hersey, N.C. Luscombe, G. Beck, B. Sherborne, I. Cooper, Pharm. Res. 19, 1446 (2002).
44. B.C. Ranu, S. Banerjee, Org. Lett. 7, 3049 (2005).
45. S.L. Fulmer, D.P. Richardson, T.E. Smith, S. Wolff, Org. Synth. 79, 236 (2002).
46. D.F. Montaño, H. Casanova, W.I. Cardona, L.F. Giraldo, Mater. Chem. Phys. 198, 386 (2017).
47. E. García, J.C. Coa, E. Otero, M. Carda, I.D. Vélez, S.M. Robledo, W.I. Cardona. Med. Chem. Res. 27, 497 (2018).
48. C.D. Sumi, B.W. Yang, I.C. Yeo, Y.T. Hahm, Can. J. Microbiol. 61, 93 (2015).
49. N. Kazuhiko, N. Ryota, O. Takashi, Jpn Dent Sci Rev 44, 29 (2008).
50. B. Yoo, B. Jing, S.E. Jones, G.A. Lamberti, Y. Zhu, J.K. Shan, E.J. Maginn, Sci. Rep. 6, 19889 (2016).
51. B.J. Denny, L. Novotny, P.W.J. West, M. Blesova, J. Zamocka, Med. Princ. Pract. 14, 377 (2005).
52. C.A. Lipinski, F. Lombardo, B.W. Dominy, P.J. Feeney, Adv. Drug Deliv. Rev. 23, 3 (1997).
53. P. Ertl, B. Rohde, P. Selzer. J. Med.Chem. 43, 3714 (2000).
54. a) N. Yang, M. Hinner, Methods Mol. Biol. 1266, 29 (2015) b) J.T. Gannon, V.B. Manilal, M. Alexander, Appl. Environ. Microbiol. 57, 190 (1991) c) K.M, Małgorzata, Z. Andrzej, R.G. Anna, L. Katarzyna, S. Leon, Microbiology 147, 2769 (2001) d) D.F. Veber, S.R. Johnson, H.Y. Cheng, B.R. Smith, K.W. Ward, K.D. Kopple. J. Med. Chem. 45, 2615 (2002).


