ZIDOVUDINE: STRUCTURAL MODIFICATIONS AND THEIR IMPACT ON BIOLOGICAL ACTIVITIES AND PHARMACOKINETIC PROPERTIES
- Human Immunodeficiency Virus (HIV),
- Hybrids,
- Phosphate and phosphonate,
- Polymers,
- Prodrugs
Abstract
Zidovudine was the first drug approved for the treatment of Acquired Immuno Deficiency Syndrome (AIDS). Its chemical name is azidothymidine (AZT). AZT
use is associated with bone marrow toxicity. Shorter half-life and inability to penetrate the blood-brain barrier are the pharmacokinetic problems of this important
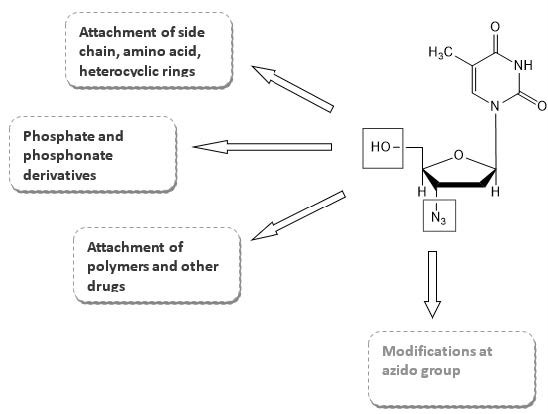
drug molecule. Various modifications have been carried out in the structure of AZT. These include modification of hydroxyl and azido group, thymidine ring,
preparation of phosphate and phosphonate derivatives, attachment of polymers and synthesis of hybrid molecules. Some of these changes produced compounds with
significantly increased activity and less toxicity. This review highlights the various structural modifications of AZT and their influence on biological activities and
pharmacokinetic properties.
References
2. M. S. Cohen, N. Hellmann, J. A. Levy, K. DeCock and J. Lange, J. Clin. Invest. 118, 1244, (2008).
3. WHO, HIV/AIDS, (2016).
4. UNAIDS Fact sheet, (2016) www.unaids.org.
5. G. R. Kaufmann and D. A. Cooper, Curr. Opin. Microbiol. 3, 508, (2000).
6. V. V. Iyer, G. W. Griesgraber, M. R. Radmer, E. J. McIntee and C. R. Wagner, J. Med. Chem. 43, 2266, (2000).
7. S. Vyas, R. Subhedar and S. Jain, J. Pharm. Pharmacol. 58, 321, (2006).
8. D. Wu, S. Ji, Y. Wu, Y. Ju and Y. Zhao, Bioorg. Med. Chem. Lett. 17, 2983, (2007).
9. I. Kostova, S. Raleva, P. Genova and R. Argirova, Bioinorg. Chem. Appl. 2006, (2006).
10. R. Ragno, S. Frasca, F. Manetti, A. Brizzi and S. Massa, J. Med. Chem. 48, 200, (2005).
11. S. Broder, Antiviral Res. 85, 1, (2010).
12. D. D. Richman, M. A. Fischl, M. H. Grieco, M. S. Gottlieb, P. A. Volberding, O. L. Laskin, J. M. Leedom, J. E. Groopman, D. Mildvan and M. S. Hirsch, New Engl. J. Med. 317, 192, (1987).
13. M. S. Abers, W. X. Shandera and J. S. Kass, CNS Drugs 28, 131, (2014).
14. M. D. Lynx and E. E. McKee, Biochem. Pharmacol. 72, 239, (2006).
15. L. M. Mansky and L. C. Bernard, J. Virol. 74, 9532, (2000).
16. D. D. Richman, Antiviral Res. 71, 117, (2006).
17. W. M. Pardridge, Mol. Interventions 3, 90, (2003).
18. J. Im, W. Kim, K.-T. Kim and S.-K. Chung, Chem. Commun., 4669, (2009).
19. H. Song, G. W. Griesgraber, C. R. Wagner and C. L. Zimmerman, Antimicrob. Agents Chemother. 46, 1357, (2002).
20. P. N. Solyev, A. V. Shipitsin, I. L. Karpenko, D. N. Nosik, L. B. Kalnina, S. N. Kochetkov, M. K. Kukhanova and M. V. Jasko, Chem. Biol. Drug Des. 80, 947, (2012).
21. C. Santos, J. Morais, L. Gouveia, E. De Clercq, C. Pannecouque, C. U. Nielsen, B. Steffansen, R. Moreira and P. Gomes, Chem. Med. Chem. 3, 970, (2008).
22. B. Pemmaraju, H. K. Agarwal, D. Oh, K. W. Buckheit, R. W. Buckheit Jr, R. Tiwari and K. Parang, Tetrahedron Lett. 55, 1983, (2014).
23. N. A. Al-Masoudi, Y. A. Al-Soud, I. A. Ali, T. Schuppler, C. Pannecouque and E. De Clercq, Nucleos. Nucleot. Nucl. 26, 223, (2007).
24. Z. You and H. J. Lee, Nucleos. Nucleot. Nucl. 25, 37, (2006).
25. G. J. Parry, C. M. Rodrigues, M. M. Aranha, S. J. Hilbert, C. Davey, P. Kelkar, W. C. Low and C. J. Steer, Clin. Neuropharmacol. 33, 17, (2010).
26. A. Dalpiaz, G. Paganetto, B. Pavan, M. Fogagnolo, A. Medici, S. Beggiato and D. Perrone, Mol. Pharm. 9, 957, (2012).
27. R. M. da Rosa, B. C. Piccoli, F. D. A. da Silva, L. Dornelles, J. B. Rocha, M. S. Sonego, K. R. Begnini, T. Collares, F. K. Seixas and O. E. Rodrigues, Med. Chem. Comm. 8, 408, (2017).
28. M. A. Raviolo, J. S. Trinchero-Hernández, G. Turk and M. C. Briñón, J. Braz. Chem. Soc. 20, 1870, (2009).
29. A.-L. Villard, G. Coussot, I. Lefebvre, P. Augustijns, A.-M. Aubertin, G. Gosselin, S. Peyrottes and C. Périgaud, Biorg. Med. Chem. 16, 7321, (2008).
30. T. Calogeropoulou, A. Detsi, E. Lekkas and M. Koufaki, Curr. Top. Med. Chem. 3, 1467, (2003).
31. A. L. Khandazhinskaya, D. V. Yanvarev, M. V. Jasko, A. V. Shipitsin, V. A. Khalizev, S. I. Shram, Y. S. Skoblov, E. A. Shirokova and M. K. Kukhanova, Drug Metab. Dispos. 37, 494, (2009).
32. P. Manda and M. Jayapal, Heterocyclic Lett. 5, 185, (2015).
33. S. S. Reddy, V. K. Rao, K. Venkataramana, C. S. Reddy, S. Ghosh and C. N. Rju, Der Pharma Chemica 2, 1, (2010).
34. K. Kolodziej, J. Romanowska, J. Stawinski, J. Boryski, A. Dabrowska, A. Lipniacki, A. Piasek, A. Kraszewski and M. Sobkowski, Eur. J. Med. Chem. 100, 77, (2015).
35. H. K. Agarwal, G. F. Doncel and K. Parang, Tetrahedron Lett. 49, 4905, (2008).
36. D. Lewandowski, M. Lewandowska, P. Ruszkowski, A. Pińska and G. Schroeder, PLoS One 10, e0126251, (2015).
37. R. Morphy and Z. Rankovic, J. Med. Chem. 48, 6523, (2005).
38. M. H. Manyeruke, T. O. Olomola, S. Majumder, S. Abrahams, M. Isaacs, N. Mautsa, S. Mosebi, D. Mnkandhla, R. Hewer and H. C. Hoppe, Biorg. Med. Chem. 23, 7521, (2015).
39. H. C. Kolb, M. Finn and K. B. Sharpless, Angew. Chem. Int. Ed. 40, 2004, (2001).
40. V. R. Sirivolu, S. K. V. Vernekar, T. Ilina, N. S. Myshakina, M. A. Parniak and Z. Wang, J. Med. Chem. 56, 8765, (2013).
41. A. J. Rao, V. K. Rao, P. V. Rao, B. S. Krishna, C. N. Raju and S. Ghosh, Int. J. Pharma Bio Sci 1, (2010).
42. D. Baraniak, K. Kacprzak and L. Celewicz, Bioorg. Med. Chem. Lett. 21, 723, (2011).
43. T. O. Olomola, R. Klein, N. Mautsa, Y. Sayed and P. T. Kaye, Biorg. Med. Chem. 21, 1964, (2013).
44. T. A. D. Thi, N. T. K. Tuyet, H. T. Nguyen, C. B. Thi, H. T. Phuong, L. Van Boi, T. Van Nguyen and M. D’hooghe, Tetrahedron Lett. 56, 218, (2015).
45. T. A. D. Thi, N. T. K. Tuyet, H. T. Nguyen, C. B. Thi, T. D. Duy, M. D’hooghe and T. Van Nguyen, Bioorg. Med. Chem. Lett. 24, 5190, (2014).
46. H.W. Zhang, M. Detorio, B. D. Herman, S. Solomon, L. Bassit, J. H. Nettles, A. Obikhod, S.J. Tao, J. W. Mellors and N. Sluis-Cremer, Eur. J. Med. Chem. 46, 3832, (2011).
47. N. Sluis-Cremer, D. Koontz, L. Bassit, B. I. Hernandez-Santiago, M. Detorio, K. L. Rapp, F. Amblard, L. Bondada, J. Grier and S. J. Coats, Antimicrob. Agents Chemother. 53, 3715, (2009).
48. B. D. Herman, R. F. Schinazi, H.-w. Zhang, J. H. Nettles, R. Stanton, M. Detorio, A. Obikhod, U. Pradere, S. J. Coats and J. W. Mellors, Nucleic Acids Res. 40, 381, (2011).
49. H.-w. Zhang, S. J. Coats, L. Bondada, F. Amblard, M. Detorio, G. Asif, E. Fromentin, S. Solomon, A. Obikhod and T. Whitaker, Bioorg. Med. Chem. Lett. 20, 60, (2010).
50. P. Singh, U. Gupta, A. Asthana and N. K. Jain, Bioconj. Chem. 19, 2239, (2008).
51. D. D. N'da and J. C. Breytenbach, J. Pharm. Pharmacol. 61, 721, (2009).
52. D. D. David, J. C. Breytenbach and J. W. Breytenbach, Arzneimittelforschung 60, 575, (2010).
53. A. Neeraj, M. Chandrasekar, U. Sara and A. Rohini, Drug Deliv. 18, 272, (2011).
54. W. Li, Y. Chang, P. Zhan, N. Zhang, X. Liu, C. Pannecouque and E. De Clercq, Chem. Med. Chem. 5,1893, (2010).
55. W. Li, J. Wu, P. Zhan, Y. Chang, C. Pannecouque, E. De Clercq and X. Liu, Int. J. Biol. Macromol. 50, 974, (2012).
56. S. Wannachaiyasit, P. Chanvorachote and U. Nimmannit, AAPS Pharm. Sci. Tech. 9, 840, (2008).
57. T. Senanayake, S. Gorantla, E. Makarov, Y. Lu, G. Warren and S. Vinogradov, Mol. Pharm. 12, 4226, (2015).
58. Y. Ahmadibeni, R. Tiwari, C. Swepson, J. Pandhare, C. Dash, G. F. Doncel and K. Parang, Tetrahedron Lett. 52, 802, (2011).
59. D. Sriram, N. Srichakravarthy, T. Bal and P. Yogeeswari, Biomed. Pharmacother. 59, 452, (2005).
60. H. Schott, K. Hamprecht, S. Schott, T. C. Schott and R. A. Schwendener, Biorg. Med. Chem. 17, 303, (2009).
61. P. Senthilkumar, J. Long, R. Swetha, V. Shruthi, R. R. Wang, S. Preethi, P. Yogeeswari, Y.T. Zheng and D. Sriram, Nucleos. Nucleot. Nucl. 28, 89, (2009).
62. P. N. Solyev, M. V. Jasko, I. L. Karpenko, Y. A. Sharkin, A. V. Shipitsyn and M. K. Kukhanova, Nucleos. Nucleot. Nucl. 33, 64, (2014).
63. C. Vanpouille, A. Khandazhinskaya, I. Karpenko, S. Zicari, V. Barreto-de-Souza, S. Frolova, L. Margolis and S. Kochetkov, Antiviral Res. 109, 125, (2014).


