SYNTHESIS, CHARACTERIZATION AND DNA INTERACTION OF CU(II) COMPLEXES WITH HYDRAZONE-SCHIFF BASE LIGANDS BEARING ALKYL QUATERNARY AMMONIUM SALTS

- hydrazone,
- quaternary ammonium salts,
- mono- and binuclear copper(II) complex,
- DNA binding and cleavage
Copyright (c) 2020 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
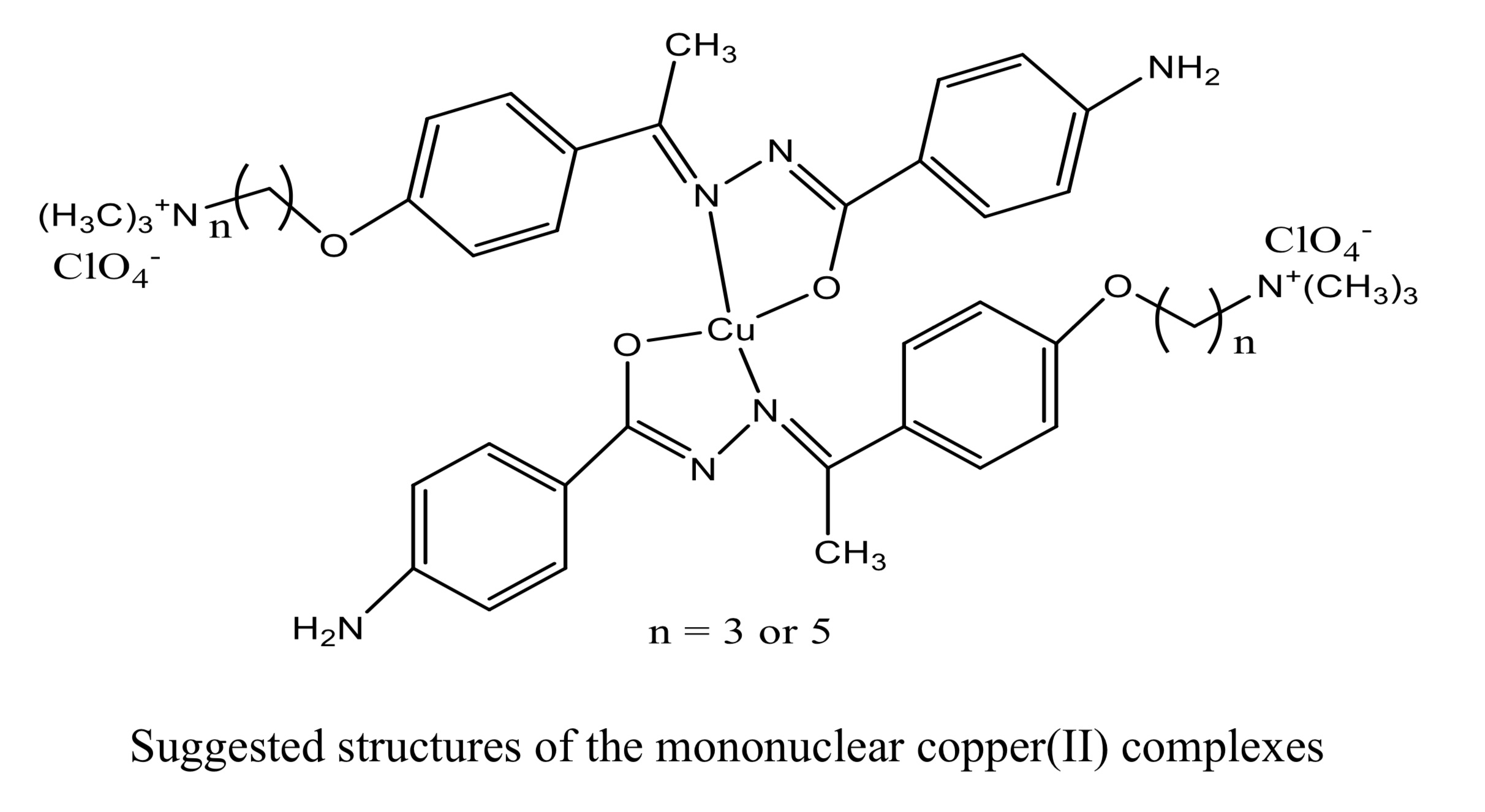
Four new Schiff-base hydrazone ligands were synthesized by the condensation of 2- and 4-aminobenzoylhydrazone with 3-(4-acetylphenoxy)-N,N,N-trimethylpropane-1-ammonium and 5-(4-acetylphenoxy)-N,N,N-trimethylpentane-1-ammonium salts. The structures of the ligands were confirmed by means of NMR, FTIR, and elemental analysis. The mononuclear and binuclear copper complexes containing these ligands were also obtained. The hydrazine-Schiff base compounds behave either as bidentate (NO sites) monobasic or tridentate (NNO sites) dibasic as ligands depending on the position of the amino group. Elemental analyses, magnetic susceptibility, FTIR and mass spectroscopy were used to confirm the proposed structures of new copper complexes. DNA binding and cleavage activities of all compounds were investigated. UV-vis spectroscopy results suggest that all compounds preferably bind to DNA via intercalation mode. According to the results of electrophoresis studies, the compounds exhibit significant cleavage activity on the plasmid DNA both in the absence and presence of hydrogen peroxide, depending on the concentration of the compounds.
References
- G. Küçükgüzel, A. Kocatepe, E. De Clercq, F. Sahin, M. Güllüce, Eur. J. Med. Chem. 41, 353, (2006).
- T. R. Todorovic, U. Rychlewska, B. Warzajtis, D. D. Radanovic, N. R. Filipovic, I. A. Pajic, D. M. Sladic, K. K Andelkovic, Polyhedron, 28, 2397, (2009).
- C. Marzano, M. Pellei, D. Colavito, S. Alidor, G. G. Lobbia, V. Gandin, F. Tisato, C. Santini, J. Med. Chem. 49, 7317, (2006).
- M. X. Li, L. Z. Zhang, C. L. Chen, J. Y. Niu, B. S. Ji, J. Inorg. Biochem. 106, 117, (2012).
- A. T. Chaviara, P. C. Christidis, A. Papageorgiou, E. Chrysogelou, D. J. Hadjipavlou-Litina, C. A. Bolos, J. Inorg. Biochem. 99, 2102, (2005).
- F. Sparatore, G. Pirisino, M. Alamanni, P. Manca-Dimich, M. Satta, Boll. Chim. Farmac. 117, 638, (1978).
- W. M. Singh, B. C. Dash, Pesticides, 22, 33, (1988).
- E. Tsuchida, K. Oyaizu, Coord. Chem. Rev. 237, 213, (2003).
- D. C. Sherrington, Chem. Soc. Rev. 28, 85, (1999).
- M. S. Shongwe, S. H. Al-Rahbi, M. A. Al-Azani, A. A. Al-Muharbi, F. Al-Mjeni, D. Matoga, A. Gismelseed, I. A. Al-Omari, A. Yousif, H. Adams, M. J. Morris, M. Mikuriya, Dalton Trans. 41, 500, (2012).
- P. Krishnamoorthy, P. Sathyadevi, K. Senthilkumar, P. T. Muthiah, R. Ramesh, N. Dharmaraj, Inorg. Chem. Commun. 14, 1318, (2011).
- S. Kathiresan, S. Mugesh, J. Annaraj, M. Murugan, New J. Chem. 41, 1267, (2017).
- T. C. Johnstone, K. Suntharalingam, S. J. Lippard, Chem. Review, 116, 3436, (2016).
- J. Kavanagh, D. Tresukosol, C. Edwards, J. Clinic. Oncology, 13, 1584, (1995).
- C. Marzano, M. Pellei, F. Tisato, C. Santini, Med. Chem. 9, 185, (2009).
- M. V. Angelusiu, S. F. Barbuceanu, C. Draghici, G. L. Almajan, Eur. J. Med. Chem. 45, 2055, (2010).
- G. Verma, A. Marella, M. Shaquiquzzaman, M. Akhtar, M. R. Ali, M. M. Alam, J. Pharm. Bioallied. Sci. 6, 69, (2014).
- R. N. Sharma, K. P. Sharma, S. N. Dikshit, Arch. Appl. Sci. Res. 3, 415, (2011).
- V. N. Telvekar, A. Belubbi, V. K. Bairwa, K. Satardekar, Bioorg. Med. Chem. Lett. 22, 2343, (2012).
- G. Tamasi, L. Chiasserini, L. Savini, A. Sega, R. Cini, J. Inorg. Biochem. 99, 1347, (2005).
- P. V. Bernhardt, P. Chin, P.C. Sharpe, J. Y. Wang, D. R. Richardson, J. Biol. Inorg. Chem. 10, 761, (2005).
- D. S. Kalinowski, P. C. Sharpe, P. V. Bernhardt, D. R. Richardson, J. Med. Chem. 51, 331, (2008).
- S. K. Sridhar, M. Saravanan, A. Ramesh, Eur. J. Med. Chem. 36, 615, (2001).
- J. Xie, S. Shen, R. Chen, J. Xu, K. Dong, J. Huang, Q. Lu, W. Zhu, T. Ma, L. Jia, H. Cai, H. Zhu, Oncol. Lett. 13, 4413, (2017).
- N. V. Shtyrlin, S. V. Sapozhnikov, A. S. Galiullina, A. R. Kayumov, O. V. Bondar, E. P. Mirchink, E. B. Isakova, A. A. Firsov, K. V. Balakin, Y. G. Shtyrlin, Biomed. Res. Int. 3864193, (2016).
- K. P. C. Minbiole, M. C. Jennings, L. E. Ator, J. W. Black, M. C. Grenier, J. E. LaDow, K. L. Caran, K. Seifert, W. M. Wuest, Tetrahedron, 72, 3559, (2016).
- I. Kowalczyk, Molecules, 13, 379, (2008).
- C. V. Kumar, E. H. Asuncion, J. Am. Chem. Soc. 115, 8547. (1993).
- P. R. Reddy, A. Shilpa, N. Raju, P. Raghavaiah, J. Inorg. Biochem. 105, 1603, (2011).
- R. Gup, B. Kırkan, Spectrochim. Acta A, 64, 809, (2006) .
- I. C. Mendes, J. P. Moreira, N. L. Speziali, A. S. Mangrich, J. A. Takahashi, H. Beraldo, J. Brazil. Chem. Soc. 17, 1571, (2006).
- C. Gokce, R. Gup, Appl. Organomet. Chem. 27, 263, (2012).
- P. Dinda, P. Sengupta, S. Ghosh, T. C. M. Mak, Inorg. Chem. 41, 1684, (2002).
- F. Tisato, C. Marzano, M. Porchia, M. Pellei, C. Santini, Med. Res. Rev. 30, 708, (2010).
- P. Krishnamoorthy, P. Sathyadevi, A. H. Cowley, R. R. Butorac, N. Dharmaraj, Eur. J. Med. Chem. 46, 3376, (2011).
- S. Tabassum, W. M. Al-Asbahy, M. Afzal, F. Arjmand, V. Bagchi, Dalton Trans. 41, 4955, (2012).
- M. F. Iskander, L. El Sayed, N. M. H. Salem, R. Werner, W. Haase, J. Coord. Chem. 56, 1075, (2003).
- R. Gup, B. Kırkan, Spectrochim. Acta A, 62, 1188, (2005).
- M. Maekawa, S. Kitagawa, Y. Nakao, S. Sakamoto, A. Yatani, W. Mori, S. Kashino, M. S. Munakata, Inorg. Chim. Acta, 293, 20, (1999).
- L. Zhi Li, C. Zhao, T. Xu, H. Wei Ji, Y. Hong Yu, G. Qiang Guo, H. Chao, J. Inorg. Biochem. 99, 1076, (2005).
- E. Lamour, S. Routier, J. L. Bernier, J. P. Catteau, C. Bailly, H. Vezin, J. Am. Chem. Soc. 121, 1862, (1999).
- K. H. Reddy, P. S. Reddy, P. R. Babu, J. Inorg. Biochem. 77, 169, (1999).
- K. Dhara, J. Ratha, M. Manassero, X. Wang, S. Gao, P. Banerjee, J. Inorg. Biochem. 101, 95, (2007).
- T. Ozawa, A. Hanaki, K. Onodera, Polyhedron, 11, 735, (1992).
- S. S. Massoud, R. S. Perkins, F. R. Louka, W. Xu, A. L. Roux, Q. Dutercq, R. C. Fischer, F. A. Mautner, M. Handa, Y. Hiraoka, G. L. Kreft, T. Bortolotto, H. Terenzi, Dalton Trans. 43, 10086, (2014).

