DETERMINATION OF REDUCING POWER AND PHYTOCHEMICAL PROFILE OF THE CHILEAN MISTLETOE "QUINTRAL" (Tristerix corymbosus (L) Kuijt) HOSTED IN “MAQUI” (Aristotelia chilensis), “HUAYÚN” (Rhaphitamnus spinosus) AND “POPLAR” (Populus nigra).

- Quintral,
- Mistletoe,
- Tristerix,
- Reducing power,
- Phytochemicals
- Mapuche ...More
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
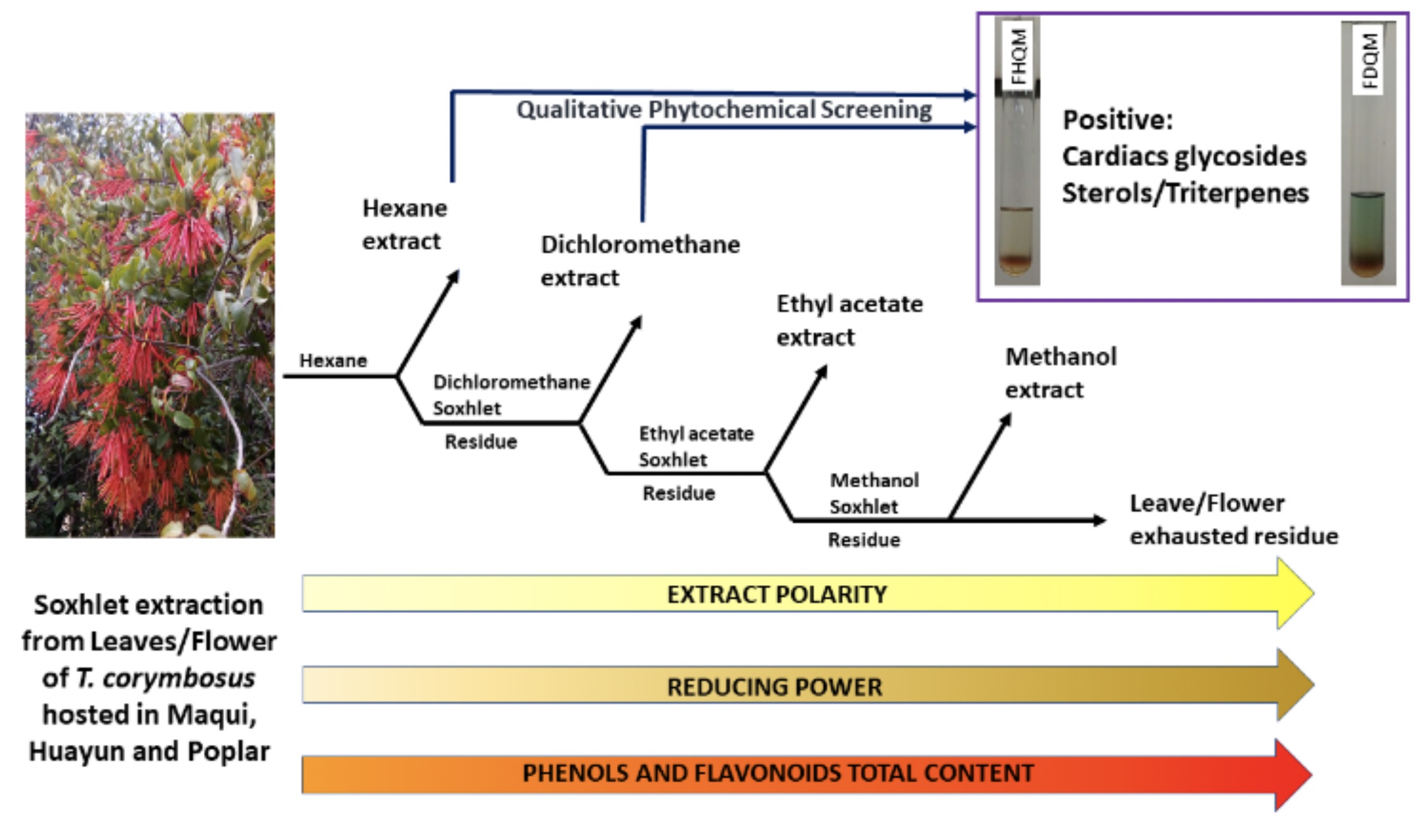
On the Chilean mistletoe “Quintral”, there has been limited literature, despite the fact the numerous uses and ubiquity amongst the Mapuche natives in Chile and Argentina. Therefore, our research group has carried out an exhaustive extraction with solvents of increasing polarity to Quintral flowers and leaves hosted in three different host species. With the extracts of different polarities, a phytochemical screening was performed, finding the presence of glycosides, sterols, terpenoids and quinones which are documented to convey different applications in cardiovascular or gastric afflictions. Likewise, the content of total phenols, total flavonoids and the reducing power of all the extracts were determined, displaying significant differences for the three varieties. Leaf and flower of the “Huayún Quintral” in methanol extracts gave the highest values of reducing power, while leaf and flower methanol extracts of “Maqui Quintral” showed the highest values for total phenols. Also observed are the lower polarity extracts from Quintral, contributing to the reducing capability by means of non-phenolic compounds.
References
- S. Bernardini, A. Tiezzi, V. Laghezza Masci, and E. Ovidi, “Natural products for human health: an historical overview of the drug discovery approaches,” Nat Prod Res, vol. 6419, no. November, pp. 1–25, 2017.
- T. D. Dillehay et al., “New archaeological evidence for an early human presence at Monte Verde, Chile,” PLoS One, vol. 10, no. 11, pp. 1–27, 2015.
- C. E. Burdick, “The remedies of the machi : visualizing Chilean medicinal botanicals in Alonso de Ovalle’s Tabula geographica (1646).,” Colon Lat Am Rev, vol. 26, no. 3, pp. 313–334, 2017.
- A. Díaz Mujica, M. V. Pérez Villalobos, C. y González Parra, and J. Simon, “Conceptos de enfermedad y sanación en la cosmovisión mapuche e impacto de la cultura occidental,” Cienc Enferm, vol. X, no. 1, pp. 9–16, 2004.
- P. J. Houghton and J. Manby, “Medicinal plants of the Mapuche,” J Ethnopharmacol, vol. 13, no. 1, pp. 89–103, Mar. 1985.
- MINSAL: Ministerio de Salud de Chile, MHT: Medicamentos Herbarios Tradicionales - 103 especies vegetales, Primera Ed. MINSAL, 2010.
- J. Kuijt, “Revision of Tristerix (Loranthaceae),” Syst. Bot. Monogr., vol. 19, no. 29, pp. 1–61, 1988.
- J. Ormeño, “Review Dodder (Cuscuta suaveolens Syr.), Mistletoe (Tristerix corymbosus L.) y Broomrape (Orobanche ramosa L.): Parasitic weeds of economic importance in Chile,” Chil J Agric Anim Sci, vol. 26, no. 2, pp. 109–119, 2010.
- D. J. Newman and M. Cragg, Gordon, “Natural Products as Drugs and Leads to Drugs: The Historical Perspective,” in Natural Product Chemistry for Drug Discoveryr, 2010, pp. 3–27.
- T. Arif et al., “Natural products - Antifungal agents derived from plants,” J Asian Nat Prod Res, vol. 11, no. 7, pp. 621–638, 2009.
- G. Appendino and F. Pollastro, “Plants: revamping the oldest source of medicines with modern science.,” in Natural Product Chemistry for Drug Discovery, 2010, pp. 140–173.
- E. European Food Safety Authority, “Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements,” EFSA Journal, vol. 10, no. 5, p. 2663. [60 pp.], 2012.
- OMS, Estrategia de la OMS sobre medicina tradicional 2014-2023. Ediciones de la OMS, 2013.
- K. Sanders, Z. Moran, Z. Shi, R. Paul, and H. Greenlee, “Natural Products for Cancer Prevention: Clinical Update 2016,” Semin. Oncol. Nurs., vol. 32, no. 3, pp. 215–240, 2016.
- S. Chikara, L. D. Nagaprashantha, J. Singhal, D. Horne, S. Awasthi, and S. S. Singhal, “Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment,” Cancer Lett, vol. 413, pp. 122–134, 2018.
- K. A. Ahmad et al., “Antioxidant therapy for management of oxidative stress induced hypertension,” Free Radic. Res, vol. 51, no. 4, pp. 428–438, 2017.
- H. N. Siti, Y. Kamisah, and J. Kamsiah, “The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review),” Vasc. Pharmacol., vol. 71, pp. 40–56, 2015.
- P. Kleniewska and R. Pawliczak, “The participation of oxidative stress in the pathogenesis of bronchial asthma,” Biomed Pharmacother, vol. 94, pp. 100–108, 2017.
- U. Asmat, K. Abad, and K. Ismail, “Diabetes mellitus and oxidative stress—A concise review,” Saudi Pharm J, vol. 24, no. 5, pp. 547–553, 2016.
- MINSAL: Ministerio de Salud de Chile, “Indicadores Basicos de Salud Chile 2015,” 2015.
- M. J. Simirgiotis, C. Quispe, C. Areche, and B. Sepúlveda, “Phenolic compounds in chilean mistletoe (quintral, Tristerix tetrandus) analyzed by UHPLC-Q/Orbitrap/MS/MS and its antioxidant properties,” Molecules, vol. 21, no. 3, pp. 1–15, 2016.
- P. Mølgaard et al., “Antimicrobial evaluation of Huilliche plant medicine used to treat wounds,” J Ethnopharmacol, vol. 138, no. 1, pp. 219–227, 2011.
- S. Chowdhury et al., “Phytochemical screening and evaluation of cytotoxic and hypoglycemic properties of Mangifera indica peels,” Asian Pac J Trop Biomed, vol. 7, no. 1, pp. 49–52, 2017.
- G. J. Kaur and D. S. Arora, “Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi,” BMC Complem Aletrn M, vol. 9, no. 30, pp. 1–10, 2009.
- R. María et al., “Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador,” J King Saud Univ Sci, no. in press, 2017.
- D. Rathee, P. Rathee, S. Rathee, and D. Rathee, “Phytochemical screening and antimicrobial activity of Picrorrhiza kurroa, an Indian traditional plant used to treat chronic diarrhea,” Arab. J. Chem., vol. 9, pp. S1307–S1313, 2016.
- S. C. Mandal, V. Mandal, and A. K. Das, “Qualitative Phytochemical Screening,” in Essentials of Botanical Extraction - Principles and applications, 1st ed., Academic Press, 2015, pp. 173–185.
- M. Saha and P. K. Bandyopadhyay, “Phytochemical screening for identification of bioactive compound and antiprotozoan activity of fresh garlic bulb over trichodinid ciliates affecting ornamental goldfish,” Aquaculture, vol. 473, pp. 181–190, 2017.
- M. T. Olivier, F. M. Muganza, L. J. Shai, S. S. Gololo, and L. D. Nemutavhanani, “Phytochemical screening, antioxidant and antibacterial activities of ethanol extracts of Asparagus suaveolens aerial parts,” S. Afr. J. Bot., vol. 108, pp. 41–46, 2017.
- H. M. Ahmed, “Phytochemical screening, total phenolic content and phytotoxic activity of corn ( Zea mays ) extracts against some indicator species,” Nat Prod Res, vol. 6419, no. November, pp. 1–5, 2017.
- A. M. Ismail, E. A. Mohamed, M. R. Marghany, F. F. Abdel-Motaal, I. B. Abdel-Farid, and M. A. El-Sayed, “Preliminary phytochemical screening, plant growth inhibition and antimicrobial activity studies of Faidherbia albida legume extracts,” J. Saudi Soc. Agric. Sci., vol. 15, no. 2, pp. 112–117, 2016.
- H. Speisky, C. López-Alarcón, M. Gómez, J. Fuentes, and C. Sandoval-Acuña, “First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the south andes region of South America,” J. Agric. Food Chem., vol. 60, no. 36, pp. 8851–8859, 2012.
- R. G. Woisky and A. Salatino, “Analysis of propolis: Some parameters and procedures for chemical quality control,” J. Apic. Res., vol. 37, no. 2, pp. 99–105, 1998.
- K. I. Berker, K. Güçlü, İ. Tor, and R. Apak, “Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents,” Talanta, vol. 72, no. 3, pp. 1157–1165, 2007.
- G. Brusotti, I. Cesari, A. Dentamaro, G. Caccialanza, and G. Massolini, “Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach,” J Pharm. Biomed, vol. 87, pp. 218–228, 2014.
- M. Diederich, F. Muller, and C. Cerella, “Cardiac glycosides: From molecular targets to immunogenic cell death,” Biochem Pharmacol, vol. 125, pp. 1–11, 2017.
- H. Todt and H. Fozzard, “Cardiac Glycosides,” in Principles of Medical Biology, vol. 8, 1997, pp. 501–518.
- R. C. Elderfield, “The chemistry of the cardiac glycosides,” Chem Rev, vol. 17, no. 2, pp. 187–249, 1935.
- H. P. Albrecht and K.-H. Geiss, “Cardiac Glycosides and Synthetic Cardiotonic Drugs,” in Ullmann’s Encyclopedia of Industrial Chemistry., Wiley-VCH Verlag GmbH & Co. KGaA, 2000, pp. 1–18.
- T. R. Sherrod, “The Cardiac Glycosides,” Hosp. Pr., vol. 2, no. 6, pp. 56–59, 1967.
- L. Menger et al., “Trial watch: Cardiac glycosides and cancer therapy,” Oncoimmunology, vol. 2, no. 2, 2013.
- F. Leitão, D. de Lima Moreira, M. Z. de Almeida, and S. Guimarães Leitão, “Secondary metabolites from the mistletoes Struthanthus marginatus and Struthanthus concinnus (Loranthaceae),” Biochem. Syst. Ecol., vol. 48, pp. 215–218, 2013.
- O. E. Ogechukwu et al., “Steroids and triterpenoids from Eastern Nigeria mistletoe, Loranthus micranthus Linn. (Loranthaceae) parasitic on Kola acuminata with immunomodulatory potentials,” Phytochem Lett, vol. 4, no. 3, pp. 357–362, 2011.
- L. Zhang et al., “Antioxidant and Anti-inflammatory Activities of Selected Medicinal Plants Containing Phenolic and Flavonoid Compounds,” J. Agric. Food Chem., vol. 59, no. 23, pp. 12361–12367, 2011.
- H. Rui-Min et al., “Comparison of Flavonoids and Isoflavonoids as Antioxidants,” J. Agric. Food Chem., vol. 57, pp. 3780–3785, 2009.
- G. D. Mogoşanu, A. M. Grumezescu, C. Bejenaru, and L. E. Bejenaru, “Natural products used for food preservation,” in Food Preservation, A. M. Grumezescu, Ed. 2017, pp. 365–411.
- M. Peña-Cerda et al., “Phenolic composition and antioxidant capacity of Ugni molinae Turcz. leaves of different genotypes,” Food Chem, vol. 215, pp. 219–227, 2017.
- M. Rubilar et al., “Extracts of maqui (Aristotelia chilensis) and murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors,” J. Agric. Food Chem., vol. 59, no. 5, pp. 1630–1637, 2011.
- S. I. Vicas, D. Rugina, and C. Socaciu, “Antioxidant Activity of European Mistletoe (Viscum album),” in Phytochemicals as Nutraceuticals - Global Approaches to Their Role in Nutrition and Health, V. Rao, Ed. InTech, 2012, pp. 115–134.
- M. J. Simirgiotis, M. Silva, J. Becerra, and G. Schmeda-Hirschmann, “Direct characterisation of phenolic antioxidants in infusions from four Mapuche medicinal plants by liquid chromatography with diode array detection (HPLC-DAD) and electrospray ionisation tandem mass spectrometry (HPLC-ESI-MS),” Food Chem., vol. 131, no. 1, pp. 318–327, 2012.
- J. D. Smith, M. C. Mescher, and C. M. De Moraes, “Implications of bioactive solute transfer from hosts to parasitic plants,” Curr. Opin. Plant Biol., vol. 16, no. 4, pp. 464–472, 2013.
- R. L. Prior, X. Wu, and K. Schaich, “Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements,” J. Agric. Food Chem., vol. 53, no. 10, pp. 4290–4302, 2005.
- K. M. Schaich and J. Xie, “Re-evaluation of the 2,2-Diphenyl-1-picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity,” J. Agric. Food Chem., vol. 62, pp. 4251–4260, 2014.
- C. López-Alarcón and A. Denicola, “Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays,” Anal. Chim. Acta, vol. 763, pp. 1–10, 2013.
- I. Pinchuk, H. Shoval, Y. Dotan, and D. Lichtenberg, “Evaluation of antioxidants : Scope , limitations and relevance of assays,” Chem. Phys. Lipids, vol. 165, no. 6, pp. 638–647, 2012.
- T.-S. Chen et al., “New Analytical Method for Investigating the Antioxidant Power of Food Extracts on the Basis of Their Electron-Donating Ability : Comparison to the Ferric Reducing / Antioxidant Power ( FRAP ) Assay,” J Agr Food Chem, vol. 58, pp. 8477–8480, 2010.
- T. Shibamoto and J.-K. Moon, “Antioxidant Assays for Plant and Food Components,” J. Agric. Food Chem., vol. 57, pp. 1655–1666, 2009.
- R. M. P. B. Costa, A. F. M. Vaz, H. S. Xavier, M. T. S. Correia, and M. G. Carneiro-da-Cunha, “Phytochemical screening of Phthirusa pyrifolia leaf extracts: Free-radical scavenging activities and environmental toxicity,” S Afr J Bot, vol. 99, pp. 132–137, 2015.

