MECHANISTIC STUDY OF A RUTHENIUM HYDRIDE COMPLEX OF TYPE [RuH(CO)(N-N)(PR3)2]+ AS CATALYST PRECURSOR FOR THE HYDROFORMYLATION REACTION OF 1-HEXENE
- DFT,
- Hydroformylation,
- Ruthenium,
- Homogeneous catalysis
Copyright (c) 2017 Sergio A. Moya, Mauricio Yáñez, Catalina Pérez, Rosa López, César Zúñiga, Gloria Cárdenas Jirón

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
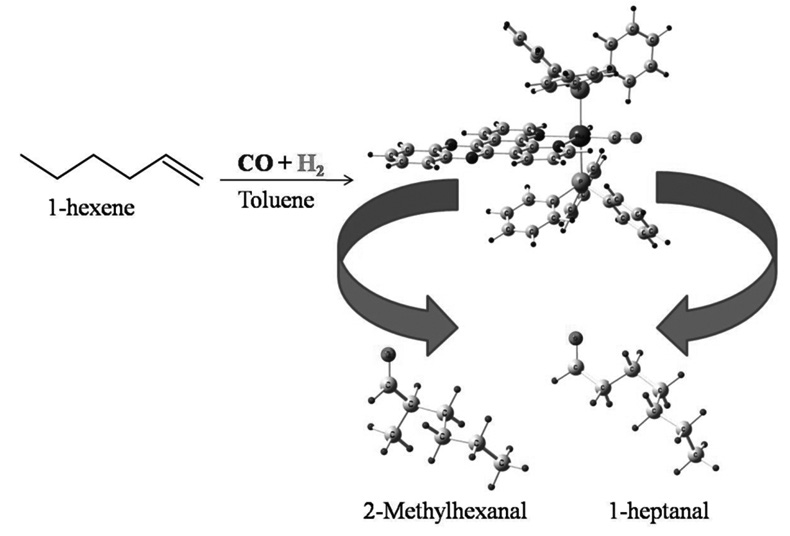
The catalytic activity of systems of type [RuH(CO)(N-N)(PR3)2]+ was evaluated in the hydroformylation reaction of 1-hexene. The observed activity is explained through a reaction mechanism on the basis of the quantum theory. The mechanism included total energy calculations for each of the intermediaries of the elemental steps considered in the catalytic cycle. The deactivation of the catalyst precursors takes place via dissociation of the polypyridine ligand and the subsequent formation of thermodynamically stable species, such as RuH(CO)3(PPh3)2 and RuH3(CO)(PPh3)2, which interrupt the catalytic cycle. In addition, the theoretical study allows to explain the observed regioselectivity which is defined in two steps: (a) the hydride migration reaction with an anti-Markovnikov orientation to produce the alkyl-linear-complex (3.1a), which is more stable by 19.4 kJ/mol than the Markovnikov orientation (alkyl-branched-complex) (3.1b); (b) the carbon monoxide insertion step generates the carbonyl alkyl-linear specie (4.1a) which is more stable by 9.5 kJ/mol than the alternative species (4.1b), determining the preferred formation of heptanal in the hydroformylation of 1-hexene.
References
- - J. Norinder, C. Rodrigues, A. Börner, J. Mol. Catal. A: Chem. 391, 139, (2014).
- - M. Madalska, P. Lönnecke, E. Hey-Hawkins, J. Mol. Catal. A: Chem. 383, 137, (2014).
- - B. Cornils, W. A. Herrmann, Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Two Volumes. 2nd ed.; Wiley, VCI-1-Weinhcim, New York: Basel: Cambridge: Tokyo, 1996.
- - K. Saikia, B. Deb, D. K. Dutta, J. Mol: Catal. A: Chem. 381, 188, (2014).
- - S. Chen, Y. Wang, W. Yao, X. Zhao, G. VO-Thanh, Y. Liu, J. Mol. Catal. A. 378, 293, (2013).
- - Y. Ohtsuka, O. Kobayashi, T. Yamakawa, J. Fluor. Chem. 161, 34, (2014).
- - S. Chen, Y. Wang, Y. Li, X. Zhao, Y. Liu, Catalysis Communications. 50, 5, (2014).
- - R. Luo, H. Liang, X. Zheng, H. Fu, M. Yuan, R. Li, H. Chen, Catalysis Communications. 50, 29, (2014).
- - R. A. Sánchez-Delgado, J. S. Bradley, G. Wilkinson, J. Chem. Soc. Dalton Trans. 399, 399, (1976).
- - R. A. Sánchez-Delgado, N. Valencia, R. Márquez-Silva, A. Andriollo, M. Medina, Inorg. Chem. 25, 1097, (1986).
- - C. U. Pittman, G. M. Wilemon, J. Org. Chem. 46, 1901, (1981).
- - V. K. Srivastava, R. S. Shukla, H. C. Bajaj, R. V. Jasra, J. Mol. Catal. A: Chem. 202, 65, (2003).
- - M. A. Moreno, M. Haukka, A. Turunen, T. A. Pakkanen, J. Mol. Catal. A: Chem. 240, 7, (2005).
- - L. Oresmaa, M. A. Moreno, M. Jakonen, S. Suvanto, M. Haukka, Applied Catalysis A General. 353, 113, (2009).
- - T. Mitsudo, N. Susuki, T. Kondo, Y. Watanabe, J. Mol. Catal. A: Chem. 109, 219, (1996).
- - M. A. Moreno, M. Haukka, M. Kailinen, T. A. Pakkanen, Appl. Organometal. Chem. 20, 51, (2006).
- - J. G. Haasnoot, W. Hinrichs, O. Weir, J. G. Vos, Inorg. Chem. 25, 4140, (1986).
- - M. Yáñez, J. Guerrero, P. Aguirre, S. A. Moya, G. Cárdenas-Jirón, J. Organomet. Chem. 694, 3781, (2009).
- - J. Ahmad, J. J. Levison, S. D. Robinson, M. F. Uttley, Inorg. Synth. 15, 45, (1974).
- - A. D. Becke, Phys. Rev. A. 38(6), 3098, (1988).
- - A. D. Becke, J. Chem. Phys. 98(7), 5648, (1993).
- - S. H. Vosko, L. Wilk, M. Nusair, Can. J. Phys. 58(8), 1200, (1980).
- - C. Lee, W. Yang, R. G. Parr, Phys. Rev. B. 37(2), 785, (1988).
- - P. J. Hay, W. R. Wadt, J. Chem. Phys. 82(1), 299, (1985).
- - TITAN 1.0.8 Wavefunction, Inc. and Schrödinger Inc. 18401 and Von Karman Avenue, Suite 370, Irvine, CA 92612 USA.
- - Jaguar 6.5, Schrödinger Inc., Portland, OR, (2005).


