- Pinostrobin,
- Structural parameters,
- Electronic structure,
- Theoretical calculations
Copyright (c) 2017 Luis Padilla Campos, Ramón A. Zarate

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
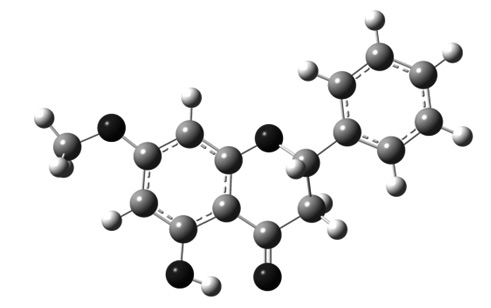
Electronic and structural properties of two polymorphic modifications of 5-hydroxy-7-methoxyflavanone (pinostrobin) were theoretically investigated and compared with experimental crystallographic data. In the literature, four polymorphic modifications of pinostrobin had been reported. The present study has established that only two of them are relevant as they differ only in the position of the methoxy group whereas other structures are derived from the rotation of the phenyl group. Both structures differ in about 1.5 kJ/mol, but the activation energy of methoxy torsion was greater than 16 kJ/mol. In addition, both have similar electronic structures but it was established with slight differences in reactivity.
References
- G.R Beecher, J. Nutr. 10, 3248S, (2003).
- J.V. Formica, W. Regelson, J. Food Chem.Toxicol. 3, 1061, (1995).
- S.N. López, M.G. Sierra, S.J. Gattuso, R.L. Furlán, S.A. Zacchino, Phytochemistry. 67, 2152, (2006).
- H.D Smolarz, E. Mendyk, A. Bogucka-Kocka, J. Kocki, Z Naturforsch C. 61, 64, (2006).
- W. Wangkangwan, S. Boonkerd, W. Chavasiri, K. Sukapirom, K. Pattanapanyasat, N. Kongkathip, T. Miyakawa, C. Yompakdee, Biosci. Biotechnol.Biochem. 73, 1679, (2009).
- A. Nix, C.A. Paull, M. Colgrave, SpringerPlus. 4, 1, (2015).
- J.W. Fahey, K.K. Stepheson, J.Agric.Food Chem. 50, 7472, (2002).
- N.A. Yusuf, M. Suffian, M. Annuar, N. Khalid, AJCS. 7, 730, (2013).
- T. Sudai, S. Prabpai, P. Kongsaeree, C. Wattapiromsakul, S. Tewtrakul, J. Ethnopaharm. 154, 453, (2014).
- N.K. Patel, K.K. Bhutani, Phytomedicine. 21, 946, (2014).
- G. Smistad G., J. Jacobsen, S.A. Sande, Int.J.Pharmac. 330, 14, (2007).
- J.S. Ashidi, P.J. Houghton, P.J. Hylands, S. Sieber, T. Efferth, Planta Medica. 73, 855, (2007).
- J.C. Le Bail, L. Aubourg, G. Habrioux , Cancer Letter. 156, 37, (2000).
- N.K. Patel, G. Jaiswal, K.K. Bhutani, Nat.Prod. Res. 30, 2017 (2016).
- A.E. Radi. S. Eissa , J. Open Chem.Biomed.Meth. 3, 74, (2010).
- K.L. Wolfe, R.H. Liu, J. Agric. Food Chem. 56, 8404, (2008).
- H. Jiye, W. Yoonkyung, H. Do-seok, J. Geunhyeong, E. Sunglock, L. Younggiu, C.P. Jun, L. Yoongho, Bioorg. Med.Chem.Lett. 20, 5510, (2010).
- C. Yaung-Hung, Y. Zhi-Shiang, W. Chi-Chung, C. Yeong-Sheng, W. Bo- Cheng, H. Chih-Ang, S. Tzenge-Lien, Food Chem. 134, 717, (2012).
- K. Hassanzadeh, K. Akhtari, H. Hassanzadeh, S. Amir-Zarei, N. Fakhraei, K.Hassanzadeh, Food Chem. 164, 251 (2014).
- R.D. Vargas-Sánchez, A.M. Mendoza-Wilson, G.R. Torrescano-Urrutia, A. Sánchez-Escalante, Comp.Theo.Chem. 1066, 7, (2015).
- E.A. Plazas, L.E. Cuca, W.A. Delgado, Rev.Colomb.Quím, 37, 135, (2008).
- M.Shoja, Z.Kristallographie. 189, 89, (1989).
- M. Shoja, Acta Cryst. C45, 828, (1989).
- V.I. Yamovoi, E.A. Kul’magambetova, A.T. Kulyyasov, K.M. Turdybekov, S.M. Adekenov, Chem.Nat.Comp. 37, 424, (2001).
- I. Brito, M.J. Simirgiotis, A. Brito, M. Rodriguez Werner, J. Borquez, P. Wintherhalter, A. Cardenas, J.Chi.Chem.Soc. 60, 2864, (2015).
- M. Head-Gordon, J.A. Pople, M.J. Frisch, Chem.Phys.Lett. 153, 503, (1988).
- S. Saebo, J. Almlöf, Chem.Phys.Lett. 154, 83, (1989).
- M.J. Frisch, M. Head-Gordon, J.A. Pople, Chem.Phys.Lett. 166, 275, (1990).
- M.J. Frisch, M. Head-Gordon, J.A. Pople, Chem.Phys.Lett. 166, 281, (1990).
- M. Head Gordon, T. Head-Gordon, Chem.Phys.Lett. 220, 122, (1994).
- A.D. Becke, J.Chem.Phys. 98, 5648, (1993).
- A.D. Becke, Phys.Rev.A. 38, 3098, (1988).
- B. Miehlich, A. Savin, H. Stoll, H. Preuss , Chem.Phys.Lett. 157, 200, (1989).
- C. Lee, W. Yang, R.G. Parr, Phys.Rev.B. 37,785, (1988).
- G.A. Petersson, A. Bennett, T.G. Tensfeldt, M.A. Al-Laham, W.A. Shirley and J. Mantzaris, J.Chem.Phys. 89, 2193, (1988).
- G.A. Petersson, M.A. Al-Lahman, J.Chem.Phys. 94, 6081, (1991).
- J. E. Carpenter, F. Weinhold, J.Mol.Struct.(Theochem). 46, 41, (1988).
- F. Weinhold, J.E. Carpenter, The Structure of Small Molecules and Ions, Plenum, New Yok, 1988.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2010.
- R.R. Contreras, P. Fuentealba, M. Garván, P. Pérez, Chem.Phys.Lett. 304, 405, (1999).
- A. Parwata, Y. Sukardiman, H.S. Mulja, A. Widhiartini, A. Gunawan, Pure Appl.Chem.Sci., 3, 19, (2015).


