SYNTHESIS, CHARACTERIZATION, AND GAS TRANSPORT PROPERTIES OF NEW COPOLY(ETHER-AMIDE)S CONTAINING BENZOPHENONE AROMATIC ISOPHTHALIC SEGMENT
- Copoly(ether-amide)s,
- Membranes,
- Permeability coefficients,
- Gas separations
Copyright (c) 2017 José Luis Santiago García, María Isabel Loría Bastarrachea, Julio Sánchez, Manuel Aguilar Vega

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
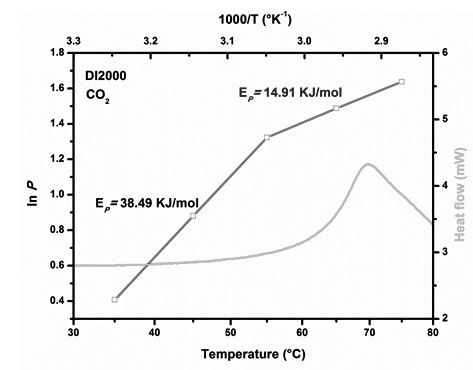
Three new copoly(ether-amide)s based on polyether segments of different molecular weight (average molecular weight 400, 900 and 2000 g/mol) and polyamide segments obtained from 4,4’-diamine benzophenone (DBF) and isophthalic acid (ISO) are reported. The resulting new copoly(ether-amide)s have inherent viscosities ranging of 0.32 to 0.35 dL/g at concentration of 0.5 g/dL, and form dense membranes by solvent casting method. The gas transport properties of the new copoly(ether-amide) membranes for pure gases (He, CO2, O2, CH4, and N2) are studied at different pressures (2.0, 5.0, 7.5, and 10.0 atm) and at different temperatures (35-75 °C). The soft segment of polyether allows an increase in gas permeability coefficients, mainly CO2, in comparison with the values reported for DBFISO aromatic polyamide. However, an increase in polyether segment length decreases the overall permeability coefficients, because the polyether shows a strong tendency to crystallize.
References
- D.F. Sanders, Z.P. Smith, R. Guo, L.M. Robeson, J.E. McGrath, D.R. Paul, B.D. Freeman, Polymer 54, 4729, (2013).
- Y. Zhang, J. Sunarso, S. Liu, R. Wang, Int. J. Greenh. Gas Con. 12, 84, (2013).
- Z. Y. Yeo, T.L. Chew, P.W. Zhu, A.R. Mohamed, S-P. Chai, J. Nat. Gas Chem. 21, 282, (2012).
- B.D. Freeman, Macromolecules 32, 375, (1999).
- A.L. Kohl and R.B. Nielsen, Gas purification, 5th Ed. Gulf Publishing Company, Houston Texas, USA, 1997.
- A. Kargon and M.T. Ravanchi, in Greenhouse gases: capturing, utiliza¬tion and reduction, G. Liu ed., InTech, 2012; chapter 1.
- B. Shimekit and H. Mukhtar, in Advances in Natural Gas Technologies, Ed. H.A. Al-Megren, InTech 2012; Chapter 9.
- H. Lin and B.D. Freeman, J. Mol. Struct. 739, 57, (2005).
- H. Lin, E.V. Wagner, B.D. Freeman, L.G. Toy, R.P. Gupta, Science 311, 639, (2006).
- J.E. Bara, C.J. Gabriel, E.S. Hatakeyama, T.K. Carlisle, S. Lessmann, R.D. Noble, D.L. Gin, J. Membr. Sci. 321, 3, (2008).
- V.A. Kusuma, B.D. Freeman, M.A. Borns, D.S. Kalika, J. Membr. Sci. 327, 195, (2009).
- H.W. Kim and H.B. Park, J. Membr. Sci. 372, 116, (2011).
- S.L. Liu, L. Shao, M.L. Chua, C.H. Lau, H. Wang, S. Quan, Prog. Polym. Sci. 38, 1089, (2013).
- J. H. Kim, S.Y. Ha, Y.M. Lee, J. Membr. Sci. 190, 179, (2001).
- H. Lin and B.D. Freeman, J. Membr. Sci. 239, 105, (2004).
- E.M. Maya, D.M. Muñoz, J.G. de la Campa, J. de Abajo, A.E. Lozano, Desalination 1999, 188, (2006).
- A. Car, C. Stropnik, W. Yave, K.V.J Peinneman, J. Membr. Sci. 307, 88, (2008).
- H. Husken, T. Visser, M. Wessling, R.J. Gaymans, J. Membr. Sci. 346, 194, (2010).
- C. Carrera-Figueiras, M. Aguilar-Vega, J. Polym. Sci. Part B: Polym. Phys. 45, 2083, (2007).
- N. Yamazaki, M. Matsumoto, F. Higashi, J. Polym. Sci. Polym. Chem. Ed. 13, 1373, (1975).
- M.I. Loría-Bastarrachea, M. Aguilar-Vega, J. Appl. Polym. Sci. 103, 2207, (2007).
- S.R. Reijerkerk, A. Arun, R.J. Gaymans, K. Nijmeijer, M. Wessling, J. Membr. Sci. 359, 54, (2010).
- M.J. van der Schuur, R.J. Gaymans, Polymer 48, 1998, (2007).
- D.M. Muñoz, E.M. Maya, J. de Abajo, J.G. de la Campa, A.E. Lozano, J. Membr. Sci. 323, 53, (2008).
- W. J. Koros, M.R. Coleman, D.R.B. Walker, Annu. Rev. Mater. Sci. 22, 47, (1992).
- S. Matteucci, Y. Yampolskii, B.D. Freeman, I. Pinnau, In Materials Science of Membranes for Gas and Vapors Separation, Yampolskii Y.; Pinnau I.; Freeman B.D. Eds., Jhon Wiley & Sons, Chichester, England, 2006, pp. 1-47.
- E. Tocci, A. Gugliuzza, L. de Lorenzo, M. Macchione, G. de Luca, Drioli E, J. Membr. Sci. 323, 316, (2008).


