- HPLC-OR,
- HPLC-CD,
- aromatic alcohols,
- optical rotation detection,
- circular dichroism detection
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
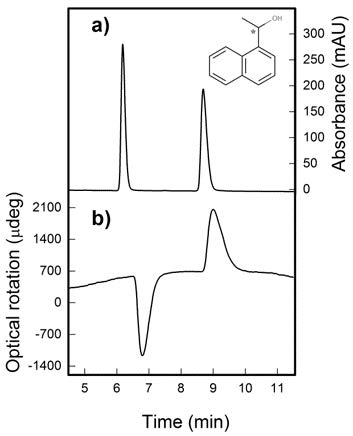
The HPLC chiral resolution of ten chiral aromatic alcohols were screened using six different polysaccharides-based chiral stationary phases (CSP) and under four normal phase conditions, using UV absorbance as detection. The screening showed that all compounds can be baseline resolved in at least one of the tested conditions, being the cellulose-methylbenzoate CSP the one with the best performance by resolving the full set. Furthermore, the use of two chiroptical detectors, based in optical rotation and electronic circular dichroism, permitted the ascertain of the elution order for each of the baseline separations. Finally, several options for a single-column batch analysis, in which samples of all compounds are tested for enantiomeric excess and absolute configuration, are proposed to analyze samples from the asymmetric reduction of the corresponding ketones. These options vary in the number of injections, analysis time and reliability, and their choice will mostly depend on the hardware and standards availability.
References
- V. Farina, J.T. Reeves, C.H. Senanayake, J.J. Song, Chem. Rev. 106, 2734–2793, (2006).
- N. Vergesson, Birth Defect Res C. 105, 140-156, (2015).
- A. Calcaterra, I. D’Acquarica, J. Pharm. Biomed. Anal. 147, 323–340, (2018).
- B. Štefane, F. Požgan, Catalysis Reviews 56, 82–174, (2014).
- B. Štefane, F. Požgan, Topics in Current Chemistry 374, 18, (2016).
- H. Shimizu, D. Igarashi, W. Kuriyama, Y. Yusa, N. Sayo, T. Saito, Org. Lett. 9, 1655-1657, (2007).
- D. Zhu, Y. Yang, L. Hua, J. Org. Chem. 71, 4202–4205, (2006).
- L. Kott, W.B. Holzheuer, M.M. Wong, G.K. Webster, J. Pharm. Biomed. Anal. 43, 57-65, (2007).
- I. Ali, H.Y. Aboul-Enein, Role of polysachccharides in chiral separations by liquid chromatography and capillary electrophoresis, in: G. Subramanian (Ed.), Chiral Separation Techniques. A Practical Approach, 3nd ed., Wiley-VCH: Weinheim, 2007; pp. 29–97.
- H.Srour, P. Le Maux, G. Simonneaux, Inorg. Chem. 51, 5850-5856, (2012).
- D.R. Li, A. He, J.R. Falck, Org. Lett. 12, 1756–1759, (2010).
- B.K. Langlotz, H. Wadepohl, L.H. Gade, Angew. Chem. Int. Ed. 47, 4670–4674, (2008).
- T. Ohkuma, M. Koizumi, H. Doucet, T. Pham, M. Kozawa, K. Murata, E. Katayama, T. Yokozawa, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 120, 13529–13530, (1998).
- Š. Vyskočil, S. Jaracz, M. Smrčina, M. Štícha, V. Hanuš, M. Polášek, P.Kočovský, J. Org. Chem. 63, 7727–7737, (1998).
- J. Chandrasekharan, P.V. Ramachandran, H.C. Brown, J. Org. Chem. 50, 5446–5448, (1985).
- M.M. Musa, K.I. Ziegelmann-Fjeld, C. Vieille, J.G. Zeikus, R.S. Phillips, J. Org. Chem. 72, 30–34, (2007).
- X. Yang, L. Su, X. Hou, S. Ding, W. Xu, B. Wang, H. Fang, J. Chromatogr. A, 1355, 291–295, (2014).


