SORPTION OF LEAD ION FROM AQUEOUS SOLUTION BY CARBOXYLIC ACID GROUPS CONTAINING ADSORBENT POLYMER
- Adsorption,
- Free Radical Polymerization,
- Malononitrile,
- Metal ions,
- p-(tetracarboxylic acid cyclopropyl) phenyl acrylate
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
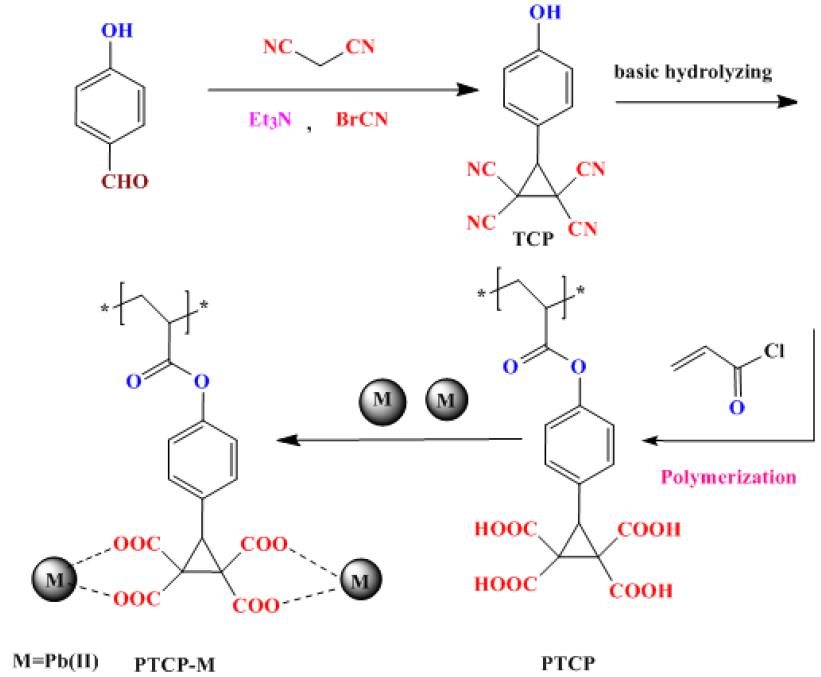
Novel method to synthesize of p-(2,2,3,3-tetracarboxylic acid cyclopropyl)phenyl acrylate(P-TCP) was disclosed. In this study,3-(4-hydroxyphenyl)cyclopropane-1,1,2,2-tetracarboxylicacid was synthesized by the new method and reacted with acryloyl chloride to preparation of P-TCP monomer. Then the resulting monomer was polymerized by free-radical polymerization initiated with benzoyl peroxide in ethyl acetate a solvent to obtain a poly-(2,2,3,3- tetra carboxylic acid cyclopropyl)phenyl acrylate (PTCP)with multicarboxylic acid cyclopropane functionalities in the pendant group as a new polymer and applied to remove Pb(II) from aqueous solution. The sorption experiments under different experimental conditions such as, contact time, temperature and pH were investigated. The functionalized polymer showed strong adsorption ability to the Pb(II), with the maximum adsorption capacities of 553 mgg-1at pH of 5.The high adsorption rate (<50 min) was seen. The removal of Pb(II) by polymer followed the pseudo-second-order rate better than the pseudo-first-order. The removal mechanisms was described as a metal-binding organic ligand (-COOH) and the electrostatic attractions between Pb(II) and oxygenic functional groups. The synthesized monomer, polymer and its metal chelates were characterized by FT-IR, 1H-NMR spectroscopy, scanning electronic microscopy (SEM) and atomic absorption techniques (AAS).
References
- A. Petrovic, M. Simonic, Int. J. Environm. Sci. Technol. 13, 1761 (2016).
- N.Isobe, X.Chen, U.Kim,S. Kimura,M. Wada,T. Saito, J. Hazard. Mater. 260,195, (2013).
- N.Lajçi, M. Sadiku, X. Lajçi, B. Baruti, S.Nikshiq, J. Int. Environ.Appl.Sci.12, 112 (2017).
- N.K.Srivastava, C.B.Majumder,J. Hazard. Mater.151,1, (2008).
- Y.Huang, X.Zeng, L.Guo, J.Lan, L.Zhang, D.Cao, Sep. Purif. Technol. 194,462, (2018).
- A. S. Raeissi, M. Shahadat, R. Bushra, S. A. Nabi, Arab. J. Sci. Eng.43, 3601, (2018).
- M. Naushad and Z.A.Alotman, Desaline.Water.Treat.J. 53,2158 , (2015).
- G.Ozkula, B.Furbano, B.Rivas, N.Kabay, M.Bryjak, J. Chil. Chem. Soc. 61,2752, (2016).
- R. Zhang,C.L.Chen,J.Li,X.K. Wang, J.Colloid Interface. Sci. 460,237, (2015).
- S. L. Luo, X.J.Li, L.Chen, J.L.Chen, Y.Wan and C.B.Liu, Chem. Eng. J. 239, 312, (2014).
- D.Zhao, Z.Zhang, H.Xuan, Y.Chen, K.Zhang,A.Alsaedi, J. Colloid Interface Sci. 506,300, (2017).
- M.Hosseinzadeh, P.Najafi Moghadam and N.NorooziPesyan, J. Polym.Mater.34,363 (2017).
- B.Sherino, S. Mohamad, N. S. Abdul Manan, H. Tareen, B. M. Yamin, S. N. Abdul Halim, Transit. Metal.Chem, 43, 53, (2018).
- B.M.Cordova, C.R.Jacinto, H. Alarcon,L.M. Mejia,R.C. Lopez, D.O. Silva, E.T.G. Caval -heiro, T. Venancio, J.Z. Davalos, A.C. Valderrama, Int. J. Biol. Macromol ,120,2259, (2018).
- Y.Huang, C.R. Li,L. Zhang, Appl. Mater.Interfaces. 6,19766, (2014).
- E.Repo, L.Malinen, R.Koivula, R.Harjula, M.Sillanpää, J. Hazard.Mater. 187,122, (2011).
- R.G.Huamani-Palomino, C. R.Jacinto, H.Alarcón, I.M.Mejía, R.C.López, D. O.Silva, E. T.G. Cavalheiro, T.Venâncio, J. Z.Dávalos, AC.Valderrama, Int. J. Biol. Macromol, In Press, https://doi.org/10.1016/j.ijbiomac.2018.09.096
- Z.S.Liu1, G.L.Rempel,Hydrol Current Res. 2,1, (2011).
- J.Faryza,K.Muhanna,D.Dari,J. Macromol. Sci., Pure Appl. Chem. 49,15,(2012).
- N.K.M.Kame, E.M.Sayyah,A.A. Abdel-aal1, Appl. Sci. Res.3,448,(2013).
- L.Cui,Y. Wang, L.Gao, L.Hu,L.Yan, Chem. Eng. J.281,1, (2015).
- J.Lee, K.Kim, A.B.Padias,Polym. Bull.31,517, (1993).
- W.W.Hartman, E.E.Dreger,Org. Synth. Coll.2,150, (1943).
- N.NorooziPesyan, M.Kimia, M.Jalilzadeh, E.J.Şahin, Chin.Chem.Soc.60, 35, (2013).
- M.Tsuda,Schotten-Baumann Esterification of Poly(viny1 alcohol). Government Chemical Industrial Research Institute, Tokyo,1963.
- C-C.Wang,C-Y Chang,C-Y. Chen,Macromol. Chem. Phys.202,882, (2001).
- R.Hasanzadeh, P.NajafiMoghadam, N.Samadi, Polym.Adv.Technol. 24,34,(2013).
- J.K.Sahoo, A.Kumar, L.Rout, J.Rath, P.Dash, H.Sahoo, Sep. Sci. Technol.53,863, (2018).
- J.Yu, J.Zheng, Q.Lu, S.Yang, X.Zhang, X. Wang, W.Yang, Colloid. Polym. Sci.294,1585, (2016).
- A. Pal, D. Das, A. K. Sarkar, S. Ghorai, R. Das, S. Pal, Eur. Polym. J.66,33, (2015).
- C.L.Chen, X.K.Wang, M.Nagatsu, Environ. Sci. Technol. 43,2362, (2009).
- C.L.Chen, J.Hu, D.D. Shao,J.X. Li, X.K. Wang, J. Hazard. Mater.164,923, (2008).


