MAGNETIC SOLID PHASE EXTRACTION APPLICATIONS COMBINED WITH ANALYTICAL METHODS FOR DETERMINATION OF DRUGS IN DIFFERENT MATRICES REVIEW
- Magnetic solid phase extraction,
- biological fluids,
- drug,
- high performance liquid chromatography,
- review
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
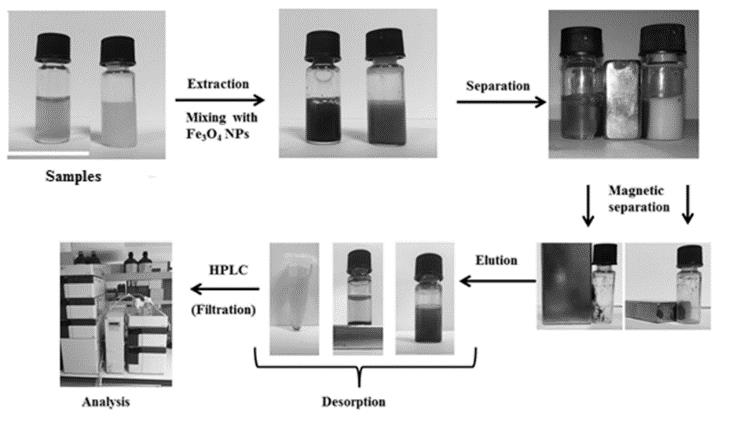
Sample preparation procedures are essential for drug analysis in biological fluids, tissues, pharmaceutical preparations, food and environmental matrices. For this purpose different techniques have been used like protein precipitation, liquid liquid extraction (LLE) and solid phase extraction (SPE). Magnetic solid phase extraction (MSPE) has become a highly preferred method due to its advantages as a sample preparation-pretreatment technique when compared to classical methods as a novel type of SPE. Basically, this type of extraction is based on the separation of different analytes from complex matrices based on the use of magnetic nanoparticles (MNPs) as adsorbents. Magnetic solid phase extraction minimizes the use of additional steps which reduces the manipulation of conventional extractions. The main advantages depend on its simplicity, environmentally friendly process, disposable cost, reduced solvent consumption, and short duration. In this review, special attention is paid on drug analysis by using MSPE prior to various analytical methods from biological, environmental and food matrices. MSPE applications, with various types of magnetic adsorbents, and different analytical method combinations were revealed for drug analysis.
Abbrevıatıons: LLE, liquid liquid extraction; SPE, solid phase extraction; MSPE, magnetic solid phase extraction; DLLME, dispersive liquid-liquid microextraction; SBSE, stir bar sorptive extraction; SPME, solid phase microextraction; SFE, supercritical fluid extraction; PFE, pressurized fluid extraction; MAE, microwave-assisted extraction; MSPD, matrix solid-phase dispersion; MNP, magnetic nanoparticle; SPION, superparamagnetic iron oxide nanoparticles; MRI, magnetic resonance imaging; CE, capillary electrophoresis; UV-Vis, ultraviolet-visible; MS, mass spectrometric; PEI, polyethyleneimine; PVA, polyvinylalcohol; PEG, polyethyleneglycol; PVP, polyvinylpyrrolidone; LC, liquid chromatography; GC, gas chromatography; HPLC, high performance liquid chromatography; FLD, fluorimetric detection; CZE, capillary zone electrophoresis; poly(MAA-co-EDMA), poly methacrylic acid co-ethyleneglycol dimethacrylate LOD, limits of detection; LOQ, limit of quantification; SDS, sodium dodecylsulfate; MPTS, 3-methacryloxypropyl trimethoxysilane; CTAB, cetyltrimethylammonium bromide; MWCNTs, multiwalled carbon nanotubes; DBMNPs, diatomite bonding Fe3O4 magnetic nanoparticles; EGDMA, ethylene glycol dimethacrylate; DCBI, desorption corona beam ionization; MNGO, magnetic nano graphene oxide; NSAIDs, nonsteroidal anti-inflammatory drugs; UAMDSPME, ultrasound-assisted magnetic dispersive solid-phase microextraction; MRLs, maximum residue limits; SA, sulfonamide; PCL, polycaprolactone; MM-PCL-SPE, microspheres solid-phase extraction; MISPE, molecularly imprinted solid-phase extraction; DMIP, dual-template molecularly imprinted polymer; MOF, metal-organic framework; ZIF-8, zeolite imidazolate framework-8; MSPDE, magnetic solid phase dispersion extraction; MIMM, molecularly imprinted magnetic microsphere.
References
- Płotka-Wasylka, J., Szczepanska, N., Guardia,M., and Namiesnik, J. (2016) J. Modern trends in solid phase extraction: New sorbent media, Trends Analyt Chem., 77:23–43.
- Andrade-Eiroa, A., Canle, M., Leroy-Cancellieri, V., and Cerdà, V. (2016) Solid-phase extraction of organic compounds: A critical review (Part I), Trends Analyt Chem., 80:641–654.
- M. Safarikova, I. Safarik, Magnetic solid-phase extraction, J. Magn. Magn.Mater 194 (1999) 108-112.
- Ito, A., Shinkai, M., Honda, H., and Kobayashi, T. (2005) Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng.,100:1-11.
- Liu, G., Men, P., Harris, PL., Rolston, R.K., Perry, G., and Smith, M.A. (2006) Nanoparticle iron chelators: a new therapeutic approach in Alzheimer disease and other neurologic disorders associated with trace metal imbalance, Neurosci. Lett., 406:189-193.
- Jalilian, A.R., Panahifar, A., Mahmoudi, M., Akhlaghi, M., and Simchi, A. (2009) Preparation and biological evaluation of 67Ga labeled superparamagnetic nanoparticles in normal rats, Radiochim. Acta, 97:51–56.
- Bulte, J.W., Douglas, T., Witwer, B., Zhang, S.C., Strable, E., Lewis, B.K., Zywicke, H., et al. (2011) Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat. Biotechnol., 19:1141-1147.
- Mahmoudi, M., Sant, S., Wang, B., Laurent, S., and Sen, T. (2011) Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy, Adv. Drug Deliver. Rev., 63:24–46.
- Sugimoto, T., and Matijevic, E. (1980) Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels, J. Colloid Interf. Sci., 174:227–243.
- Maity, D., Choo, S.G., Yi, J.B., Ding, J., and Xue, J.M. (2009) Synthesis of magnetite nanoparticles via a solvent-free thermal decomposition route, J. Magn. Magn. Mater., 321:1256–1259.
- Grzeta, B., Ristic, M., Nowik, I., and Music, S. (2002) Formation of nanocrystalline magnetite by thermal decomposition of iron choline citrate, J. Alloy. Compd., 334:304–312.
- Fievet, F., Lagier, J.P., Blin, B., Beaudoin, B., and Figlarz, M. (1989)Homogeneous and heterogeneous nucleations in the polyol process for thepreparation of micron and submicron size metal particles, Solid State Ionics, 32:198205.
- Si, S.F., Li, C.H., Wang, X., Yu, D., Peng, Q., and Li, Y.D. (2005) Magnetic monodisperse Fe3O4 nanoparticles, Cryst. Growth Design, 5:391–393.
- Durmus, Z., Baykal, A., Kavas, H., Direkçi, M., and Toprak, M.S. (2009) Ovalbumin mediated synthesis of Mn3O4, Polyhedron, 28:2119-2122.
- Muller, B.W., and Muller, R.H. (1984) Particle-size distributions and particle-size alterations in microemulsions, J. Pharm. Sci., 73:919–922.
- Alvarez, G.S., Muhammed, M., Zagorodni, A. (2006) Novel flow injection synthesis of iron oxide nanoparticles with narrow size distribution, Chem. Eng. Sci., 61:4625.
- Denouden, C.J.J., and Thompson, R.W. (1991) Analysis of the formation of monodisperse populations by homogeneous nucleation, J. Colloid Interf. Sci., 143:77–84.
- Baykal, A., Kavas, H., Durmus, Z., Demir, M., Kazan, S., Topkaya, R., and Toprak, M.S. (2010) Sonochemical synthesis and chracterization of Mn3O4 nanoparticles, Cent. Eur. J. Chem., 8:633–638.
- Pascal, C., Pascal, J.L., Favier, F., Moubtassim, M.L.E., and Payen, C. (1999) Electrochemical synthesis for the control of gamma-Fe2O3 nanoparticle size.Morphology, microstructure, and magnetic behavior, Chem. Mater., 11:141–147.
- Khollam, Y.B., Dhage, S.R., Potdar, H.S., Deshpande, S.B., Bakare, P.P., Kulkarni, S.D., and Date, S.K. (2002) Microwave hydrothermal preparation of submicron-sized spherical magnetite (Fe3O4) powders, Mater. Lett., 56:571–577.
- Bharde, A., Rautaray, D., Bansal, V., Ahmad, A., Sarkar, I., Yusuf, S.M., Sanyal, M., et al. (2006) Extracellular biosynthesis of magnetite using fungi, Small 2:135–141.
- Kim, J., Kim, H.S., Lee, N., Kim, T., Kim, H., Yu, T., Song, I.C., et al. (2008) Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery, Angew. Chem. Int. Edit., 47:8438–8441.
- Tang, D., Yuan, R., and Chai, Y. (2006) Magnetic Core−Shell Fe3O4@Ag Nanoparticles Coated Carbon Paste Interface for Studies of Carcinoembryonic Antigen in Clinical Immunoassay, J. Phys. Chem. B, 110:11640–11646.
- Pariti, A., Desai, P., Maddirala, S.K.Y., Ercal, N., Katti, K.V., Liang, X., and Nath, M. (2014) Superparamagnetic Au-Fe3O4 nanoparticles: one-pot synthesis, biofunctionalization and toxicity evaluation, Mater. Res. Express, 1:035023.
- Bartolozzi, C., Lencioni, R., Donati, F., and Cioni, D. (1999) Abdominal MR: liver and pancreas, Eur. Radiol., 9:1496–1512.
- Meng, J., Fan, J., Galiana, G., Branca, R.T., Clasen, P.L., Ma, S., Zhou, J., et al. (2009) LHRH-functionalized superparamagnetic iron oxide nanoparticles for breast cancer targeting and contrast enhancement in MRI, Mater. Sci. Eng. C, 29:1467–1479.
- Horak, D., Babic, M., Jendelova, P., Herynek, V., Trchová, M., Pientka, Z., Pollert E., et al. (2007) D-mannose-modified iron oxide nanoparticles for stem cell labeling, Bioconjug. Chem., 18:635–644.
- Andra, W., d’Ambly, C.G., Hergt, R., Hilger, I., and Kaiser, W.A. (1999) Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia, J. Magn. Magn. Mater., 194:197–203.
- Lu, W., Ling, M., Jia, M., Huang, P., Li, C., and Yan, B. (2014) Facile synthesis and characterization of polyethylenimine-coated Fe3O4 superparamagnetic nanoparticles for cancer cell separation, Mol. Med. Rep., 9:1080-1084.
- Grumezescu, V., Holban, A.M., Grumezescu, A.M., Socol, G., Ficai, A., Vasile, B.S., Truscă, R., et al. (2014) Usnic acid-loaded biocompatible magnetic PLGA-PVA microsphere thin films fabricated by MAPLE with increased resistance to staphylococcal colonization, Biofabrication, 6:035002.
- Yang, J., Zou, P., Yang, L., Cao, J., Sun, Y., Han, D., Yang, S., et al. (2014) A comprehensive study on the synthesis and paramagnetic properties of PEGcoated Fe3O4 nanoparticles, Appl. Surf. Sci., 303:425–432.
- Zhang, Y., Liu, J.Y., Ma, S., Zhang, Y.J., Zhao, X., Zhang, X.D., and Zhang, Z.D. (2010) Synthesis of PVP-coated ultra-small Fe3O4 nanoparticles as a MRI contrast agent. J. Mater. Sci. Mater. Med., 21:1205–1210.
- Trends in Analytical Chemistry 59 (2014) 50–58.
- Zhu, L., Pan, D., Ding, L., Tang, F., Zhang, Q., Liu, Q., and Yao, S. (2010) Mixed hemimicelles SPE based on CTAB-coated Fe3O4/SiO2 NPs for the determination of herbal bioactive constituents from biological samples, Talanta, 80:1873–1880.
- Chen, M.L., Suo, L.L., Gao, Q., and Feng, Y.Q. (2011) Determination of eight illegal drugs in human urine by combination of magnetic solid-phase extraction with capillary zone electrophoresis, Electrophoresis, 32:2099–2106.
- Su, S.W., Liao, Y.C., and Whang, C.W. (2012) Analysis of alendronate in human urine and plasma by magnetic solid-phase extraction and capillary electrophoresis with fluorescence detection, J. Sep. Sci., 35:681–687.
- Ahangar, L.E., Movassaghi, K., Bahrami, F., Enayati, M., and Chianese, A. (2014) Determination of Drugs in Biological Sample by Using Modified Magnetic Nanoparticles and HPLC, Chem. Engineer. Trans., 38:391-395.
- Afkhami, A., Saber-Tehrani, M., and Bagheri, H. (2010) Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution, Desalination, 263:240–248.
- Madrakian, T., Afkhami, A., Rahimi, M., Ahmadi, M., and Soleimani, M. (2013) Preconcentration and spectrophotometric determination of oxymetholone in the presence of its main metabolite (mestanolone) using modified maghemite nanoparticles in urine sample, Talanta, 115:468–473.
- Mahdavian, A.R., and Mirrahimi, M.S. (2010) Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification, Chem. Eng. J., 159:264–271.
- Panahi, H.A., Soltani, E.R., Moniri, E., Tamadon, (2013) A. Synthesis and characterization of poly[1-(N,N-bis-carboxymethyl) amino-3-allylglycerolco- dimethylacrylamide]grafted to magnetic nano-particles for extraction and determination of letrozole in biological and pharmaceutical samples, Talanta, 117:511–517.
- Behbahani, M., Bagheri, S., Amini, M.M., Abandansari, H.S., Moazami, H.R., Bagheri, A. (2104) Application of a magnetic molecularly imprinted polymer for the selective extraction and trace detection of lamotrigine in urine and plasma samples, J Sep Sci., 37:1610-1616.
- Mashhadizadeh, M.H., and Amoli-Diva, M. (2013) Atomic absorption spectrometric determination of Al3+ and Cr3+ after preconcentration and separation on 3-mercaptopropionic acid modified silica coated-Fe3O4 nanoparticles, J. Anal. Atomic Spectrom., 28:251–258.
- Pourghazi, K., and Amoli-Diva, M. (2104) Magnetic nanoparticles solid phase extraction based on the formation of supramolecular mixed hemimicelle aggregates for the determination of naproxen in biological fluids using high-performance liquid chromatography-UV, IET Micro & Nano Letters, 9:577-581.
- Shen, J., Huang, W., Wu, L., Hu, Y., and Ye, M. (2007) Study on aminofunctionalized multiwalled carbon nanotubes, Mater. Sci. Eng. A, 464:151–156.
- Daneshvar Tarigh, G., and Shemirani, F. (2013) Magnetic multi-wall carbón nanotube nanocomposite as an adsorbent for preconcentration and determination of lead (II) and manganese (II) in various matrices, Talanta, 115:744–750.
- Bigdelifam, D., Mirzaei, M., Hashemi, M., Amoli-Diva, M., Rahmani, O., Zohrabi, P., Taherimaslak, Z., et al. (2014) Sensitive spectrophotometric determination of fluoxetine from urine samples using charge transfer complex formation after solid phase extraction by magnetic multiwalled carbon nanotubes, Anal. Methods, 6:8633-8639.
- Zhang, H., and Shi, Y. (2012) Preparation of Fe3O4 nanoparticle enclosure hydroxylated multi-walled carbon nanotubes for the determination of aconitines in human serum samples, Anal. Chim. Acta, 724:54–60.
- Zhang, S.L., Cui, Y.H., Sun, J.C., Xi, Y.Q., Zhang, C.X., and Tang, J.H., (2015) Sensitive magnetic solid-phase microextraction based on oxide multiwalled carbon-nanotubes for the determination of methylamphetamine and ketamine in human urine and blood, Anal. Methods, 7:4209-4215.
- Madrakian, T., Haryani, R., Ahmadi, M., and Afkhami, A., (2015) Spectrofluorometric determination of venlafaxine in biological samples after selective extraction on the superparamagnetic surface molecularly imprinted nanoparticles, Anal. Methods, 7:428-435.
- Ebrahimi, M., Ebrahimitalab, A., Eshaghi, Z., Mohammadinejad, A., (2015) Magnetized Silane-Coupling Agent KH-570 Based Solid-Phase Extraction Followed by Gas Chromatography-Flame Ionization Detection to Determine Venlafaxine in Human Hair and Aqueous Environmental Samples, Arch. Environ. Contam. Toxicol., 68:412-420.
- Chen, D., Zheng, H.B., Huang, Y.Q., Hu, Y.N., Yu, Q.W., Yuan, B.F., and Feng, Y.Q. (2015) Magnetic solid phase extraction coupled with desorption corona beam ionization-mass spectrometry for rapid analysis of antidepressants in human body fluids, Analyst, 140:5662-5670.
- Ahmadi, M., Madrakian, T., and Afkhami A. (2016) Solid phase extraction of amoxicillin using dibenzo-18-crown-6 modified magnetic-multiwalled carbon nano tubes prior to its spectrophotometric determination, Talanta, 148:122–128.
- Madrakian, T., Fazl, F., Ahmadi, M., and Afkhami, A., (2016) Efficient solid phase extraction of codeine from human urine samples using a novel magnetic molecularly imprinted nanoadsorbent and its spectrofluorometric determination, New J. Chem., 40:122-129.
- Chen, D., Zheng, H.B., Huang, Y.Q., Hu, Y.N., Yu, Q.W., Yuan, B.F., and Feng, Y.Q. (2015) Magnetic solid phase extraction coupled with desorption corona beam ionization – mass spectrometry for rapid analysis of antidepressants in human body fluids, Analyst, 140:5662-5670.
- Zeng, S., Gan, N., Weideman-Mera, R., Cao, Y., Li, T., and Sang, W. (2013) Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide, Chem. Eng. J. 218:108.
- Taghvimi, A., Hamishehkar, H., and Ebrahimi, M. (2016) The application of magnetic nano graphene oxide in determination of methamphetamine by high performance liquid chromatography of urine samples, J. Iran. Chem. Soc., 13:1471–1480.
- Taghvimi, A., Hamishehkar, H., and Ebrahimi, M. (2016) Magnetic nano graphene oxide as solid phase extraction adsorbent coupled with liquid chromatography to determine pseudoephedrine in urine samples, J. Chromatogr. B, 1009:66–72.
- Bykkam, S., Rao, K., Chakra, C., and Thunugunta, T. (2013) Synthesis and characterization of graphene oxide and its antimicrobial activity against klebseilla and staphylococcus, Int. J. Adv. Biotechnol. Res., 4:1005–1009.
- Zeng, S., Gan, N., Weideman-Mera, R., Cao, Y., Li, T., and Sang, W. (2013) Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide, Chem. Eng. J., 218:108–115.
- Taghvimi, A., Hamishehkar, H., and Ebrahimi, M. (2016) Development and validation of a magnetic solid-phase extraction with high-performance liquid chromatography method for the simultaneous determination of amphetamine and methadone in urine, J Sep Sci., 39:2307-2312.
- Xue, X., Yang, D., Wang, D., Xu, X., Zhu, L., and Zhao, Z. (2015) Solidification of floating organic drop liquid-phase microextraction cell fishing with gas chromatography–mass spectrometry for screening bioactive components from Amomum villosum Lour, Biomed. Chromatogr., 29:626–632.
- Tankiewicz, M., Morrison, C., and Biziuk, M. (2013) Application and optimization of headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–flame-ionization detector (GC–FID) to determine products of the petroleum industry in aqueous samples, Microchem. J., 108:117–123.
- Ghorbani, M., Chamsaz, M., Rounaghi, and G.H. (2016) Ultrasound-assisted magnetic dispersive solid-phase microextraction: A novel approach for the rapid and efficient microextraction of naproxen and ibuprofen employing experimental design with high-performance liquid chromatography, J. Sep Sci., 39:1082-1089.
- Maham, M., and Sharifabadi, M.K., (2016) Simultaneous determination of trace amounts of anti-hypertensive drugs in urine using magnetic mixed hemimicelles solid-phase extraction combined with HPLC-UV, J. Anal. Chem., 71:302-309.
- Tang, M., Wang, Q., Jiang, M., Xu, L., Shi, Z.G., Zhang, T., and Liu, Y. (2014) Magnetic solid-phase extraction based on methylcellulose coated-Fe3O4–SiO2–phenyl for HPLC–DAD analysis of sildenafil and its metabolite in biological samples, Talanta, 130:427–432.
- Wu, J., Zhao, H., Chen, R., Pham-Huy, C., Hui, X., and He, H. (2016) Adsorptive removal of trace sulfonamide antibiotics by water-dispersible magnetic reduced graphene oxide-ferrite hybrids from wastewater, J. Chromatogr. A, 1029–1030:106–112.
- Wang, Y., Liu, L., Xiao, C., Chen, L., Yang, P., Liu, Q., Wang J., et al. (2016) Rapid Determination of Trace Sulfonamides in Milk by Graphene Oxide-Based Magnetic Solid Phase Extraction Coupled with HPLC–MS/MS, Food Anal. Method., 9: 2521.
- Socas-Rodríguez, B., Hernández-Borges, J., Salazar, P., Martín, M., and Rodríguez-Delgado M.Á. (2015) Core–shell polydopamine magnetic nanoparticles as sorbent inmicro-dispersive solid-phase extraction for the determination ofestrogenic compounds in water samples prior to highperformanceliquid chromatography–mass spectrometry analysis, J. Chromatogr. A, 1397:1–10.
- Haoa, Y., Gao, R., Shia, L., Liu, D., Tang, Y., and Guo, Z. (2015) Watercompatible magnetic imprinted nanoparticles served assolid-phase extraction sorbents for selective determination of trace 17beta-estradiol in environmental water samples by liquid chromatography, J. Chromatogr. A, 1396:7–16.
- Konoz, E., Sarrafi, A.H.M., and Sahebi, H. (2016) Preconcentration and determination of ranitidine hydrochloride in real samples by using modified magnetic iron oxide nanoparticles, Canad. J. Chem., 94:9-14.
- Wang, L., Xu, X., Zhang, Z., Zhang, D., Liu, X., and Zhang, L. (2015) Green sample clean-up based on magnetic multiwalled carbon nanotubes for the determination of lamivudine by high performance liquid chromatography, RSC Adv., 5:22022–22030.
- Capriotti, A.L., Cavaliere, C., La Barbera, G., Piovesana, S., Samperi, R., Chiozzi, R.Z., Lagana, A., (2016) Polydopamine-coated magnetic nanoparticles for isolation and enrichment of estrogenic compounds from surface water samples followed by liquid chromatography-tandem mass spectrometry determination, Anal. Bioanal. Chem., 408:4011-4020.
- Wang, H., Liu, S.Y., Lv, X.J., Rui, M., and Zhang, Z.Q. (2015) Assembly of a Fe–pamoate porous complex on magnetic microspheres for extraction of sulfonamide antibiotics from environmental water samples, Anal. Methods, 7:4939.
- Es'haghi, Z., Nezhadali, A., Khatibi, A.D., (2016) Magnetically responsive polycaprolactone nanoparticles for progesterone screening in biological and environmental samples using gas chromatography, Anal. Bioanal. Chem., 408:5537-5549.
- Castillo-García, M.L., Aguilar-Caballos, M.P., and Gómez-Hens, A. (2015) A europium- and terbium-coated magnetic nanocomposite as sorbentin dispersive solid phase extraction coupled with ultra-high performance liquid chromatography for antibiotic determination in meat samples, J. Chromatogr. A, 1425:73–80.
- Tian, M., Zou, Y., Zhou, S., Wang, T., Lv, X., Qiong J. (2015) Development of novel magnetic solid phase extraction materials based on Fe3O4/SiO2/poly(acrylamide-N,N-methylenebisacrylamide)-Pluronic L64 composite microspheres and their application to the enrichment of natamycin, J. Chromatogr. B, 1007:1–7.Li, Y., Wu, X., Li, Z., Zhong, S., Wang, W., Wang, A., and Chen, J. (2015) Fabrication of CoFe2O4–graphene nano composite and its application in the magnetic solid phase extraction of sulfonamides from milk samples, Talanta, 144: 1279–1286.
- Li, Y., Wu, X., Li, Z., Zhong, S., Wang, W., Wang, A., and Chen, J. (2015) Fabrication of CoFe2O4–graphene nano composite and its application in the magnetic solid phase extraction of sulfonamides from milk samples, Talanta, 144: 1279–1286.
- Karami-Osboo, R., Miri, R., Javidnia, K., Shojaee, M.H., Kobarfard, F. (2016) Extraction and determination of sulfadiazine and sulfathiazole in milk using magnetic solid phase extraction-HPLC-UV, Anal. Methods, 7:1586-1589.
- Wei, S., Li, J., Liu, Y., Jinkui Ma, J. (2016) Development of magnetic molecularly imprinted polymers withdouble templates for the rapid and selective determination ofamphenicol antibiotics in water, blood, and egg samples, Journal of Chromatography A, 1473:19–27.
- He, X., Wang, G.N., Yang, K., Liu, H.Z., Wu, X.J., Wang, J.P. (2017) Magnetic graphene dispersive solid phase extraction combining high performance liquid chromatography for determination of fluoroquinolones in foods, Food Chemistry, 221:1226–1231.
- Lan, H., Gan, N., Pan, D., Hu, F., Li, T., Long, N., Shen, H., Feng, Y. (2014) Development of a novel magnetic molecularly imprinted polymercoating using porous zeolite imidazolate framework-8 coatedmagnetic iron oxide as carrier for automated solid phasemicroextraction of estrogens in fish and pork samples, Journal of Chromatography A, 1365:35–44.
- Zheng, H., Mo, J., Zhang, Y., Gao, Q., Ding, J., Yu, Q.W., Yu-Qi Feng, Y. (2014) Facile synthesis of magnetic molecularly imprinted polymers and its application in magnetic solid phase extraction for fluoroquinolones in milk samples, Journal of Chromatography A, 1329: 17–23.
- Gao, R., Cui, X., Hao, Y., Zhang, L., Liu, D., Tang, Y. (2016) A highlyefficient imprinted magnetic nanoparticle for selective separation and detection of 17β-estradiol in milk, Food Chemistry 194:1040–1047.
- Yv, Y.K., Zhao, C.X., Li, P., He, Y.D., Yang, Z.R., Sun, H.W. Preparation of doxycycline-imprinted magnetic microspheres by inverse-emulsion suspension polymerization for magnetic dispersion extraction of tetracyclines from milk samples, Journal of Separation Science, doi:10.1002/jssc.201300429.


