REMOVAL OF CR(VI) BY STABILIZED SOLVENT IMPREGNATED RESIN (SIR) PREPARED BY USING A HYDROPHILIC POLYMER ADSORBENT AND ALIQUAT 336
- Ion exchange resins,
- solvent impregnated resin (SIR),
- Chromium,
- Adsorption
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
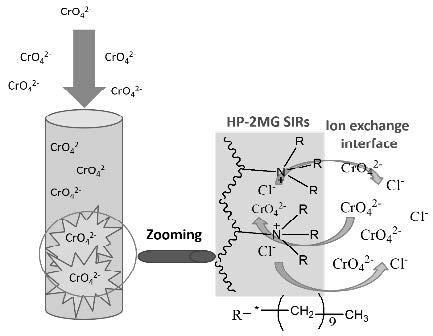
The solvent impregnated resin (SIR) was prepared by using Diaion HP-2MG as a hydrophilic polymer adsorbent and commercial Aliquat 336 as extractant for hexavalent chromium Cr(VI) removal from aqueous solution. The resulting SIRs were stabilized by coating using poly(vinyl alcohol) (PVA) and divinylsulfone as crosslinking reagent with different amounts.
In order to predict the mechanism involved in the adsorption process, several kinetic models were used. Among them, the sorption kinetics was usually described by pseudo-first or pseudo-second order models. The kinetic behavior of stabilized SIRs was investigated as a function of amount of crosslinking reagent by batch adsorption equilibrium. Uncoated resins exhibited a faster kinetics than coated ones. It was possible to improve the kinetic performance of crosslinked resins with conditioning by using NaOH-NaCl mixture. The breakthrough profiles of SIRs were also influenced by amount of crosslinking reagent.
References
- Yadav, A., Rajhans, K.P., Ramteke, S., Sahu, B.L., Patel, K.S., Blazhev, B., J. Env. Protect., 2016, 7, 72-81.
- Sari E., Cukrov N., Francikovic-Bilinki S., Kurt M.A., HalliM., Environ. Earth Sci., 2016,75:1051-1058.
- Sandhya M., Ram N. B., J. Env. Sci. Health, 2016, Part C, 34, 1, 1-32.
- World Health Organization, Guidelines for drinking-water Quality, 4th edition, Switzerland, 2011, Chapter 12, pp 340.
- Santander I. P., Rivas B.L, Urbano B.F., Leiton L., Yılmazİpek I., Yüksel M., Kabay N., Bryjak M., Polym. Bull., 2014, 71, 1813-1821.
- Rivas B.L., H.A. Maturana, Pereira E.D. Angew. Makromol. Chem. 1994, 220, 61-74.
- Rivas B.L., Pereira E. D., Palencia M., Sánchez J., Progr. Polym. Sci., 2011, 36, 2, 294-322.
- Sánchez J., Toledo L., Rivas B.L., Rivera N., Muñoz E., J. Chil. Chem. Soc., 2013, 58, 4, 1986-1993.
- Rivas B.L., Pereira E:D. Bol. Soc. Chil. Quim., 2000, 45, 165-171.
- Tapiero Y., Rivas B. L., Sánchez J., J. Chil. Chem. Soc., 2014, 59, 2737-2746.
- Tapiero Y., Sánchez J., Rivas B.L., Chinese J. Chem. Eng., 2017, 25, 938-946.
- Van Nguyen N., Lee J-C., Jeong J., Pandey B. D., Chem. Eng. J, 2013, 219, 174-182.
- Geckeler K.E., Zhou R.N., Rivas B.L., Angew. Makromol. Chem. 1992, 197, 107-115.
- Serarols J., Poch J., Villaescusa I, React. Funct.Polym., 2001, 48, 37-51.
- Kabay N., Demircioglu M., Ekinci H., Yuksel M., Saglam M., Streat M., React.Funct.Polym., 1998, 38, 219-226.
- Rivas B.L.; Moreno-Villoslada I. Chem. Lett. 2000, 116-167.
- Cortina J.L., Miralles N., Sastre A.M., Aguilar M., React. Funct.Polym., 1997, 32, 221-229.
- Kabay, N., Cortina, J.L., Trochimczuk, A., Streat, M., React. Funct.Polym., 2010, 70, 484-496.
- Kabay, N., Arda, M., Saha, B., Streat, M., React. Funct. Polym., 2003, 54, 103-115.
- Saha B., Gill R.J., Bailey D.G., Kabay N., Arda M., React. Funct. Polym., 2014, 60, 223-244.
- Trochimczuk, A.W., Kabay, N., Arda, M., Streat, M., React. Funct. Polym., 2004, 59, 1-7.
- Kabay, N., Arda, M., Trochimczuk, A., Streat, M., React. Funct. Polym., 2004, 59, 9-14.
- Kabay, N., Arda, M., Trochimczuk, A., Streat, M., React. Funct. Polym., 2004, 59, 15-22.
- Kabay, N., Solak, O., Arda, M., Topal, U., Yuksel, M., Trochimczuk, A., Streat, M., React. Funct. Polym., 2005, 64, 75-82.
- Hosseini-Bandegharaei A.,Hosseini M. S., Sarw-GhadiM., Zowghi S., HosseiniE., Hosseini-Bandegharaeia H., Chem. Eng. J., 2010, 160, 190-198.
- Qureshia I., Memon S.,Yilmaz M., J. Haz. Mat., 2009, 169, 675-682.
- Yang X-Y., Zhang J-P., Guo L., Zhao H., Zhang Y., Chen J., Trans. Non ferrous Mec. Soc. China, 2012, 22, 3126-3130.
- Soylak M., Tuzen M., Mendil D., Turkekul I., Talanta, 2006, 70, 1129-1135.
- Ghaedi M., Montazerozohori M., Haghdoust S., Zaare F., Soylak M., HET, 2013, 32 (4), 371-378.
- Duran C., Bulut V. N., Gundogdu A., Soylak M., Belduz A. O., Beris F. S., Sep. Sci. Tech., 2009, 44, 335-338.
- Demircioğlu, M., Kocacık, N., Yiğit, E., Kabay N., Innovations in Mineral and Coal Processing, 1998, 781-785.
- Kabay, N., Sarp, S,, Yüksel, M.,Arar, Ö., BryjakM, React. Funct. Polym., 2007, 67, 1643-1650.
- Vincent, T., Guibal, E., Ind. Eng. Chem. Res., 2001, 40(5), 1406-1411.
- Sanchez, J., Espinosa, C., Pooch, F., Tenhu, H., Pizarro, G. C., Oyarzun, D., React. Funct. Polym., 2018, 127, 67-73.
- Sengupta A.K., D. Clifford, Environ. Sci. Technol., 1986, 20, 153-1.


