STUDY OF THE CATALYTIC CONVERSION AND ADSORPTION OF ABIETIC ACID ON ACTIVATED CARBON: EFFECT OF SURFACE ACIDITY
- Activated carbón,
- Abietic acid,
- Tall oil,
- Adsorption capacity
Copyright (c) 2017 Rafael García, Lorena Peralta, Cristina Segura, Catherine Sepúlveda, I. Tyrone Ghampson, Nestor Escalona

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
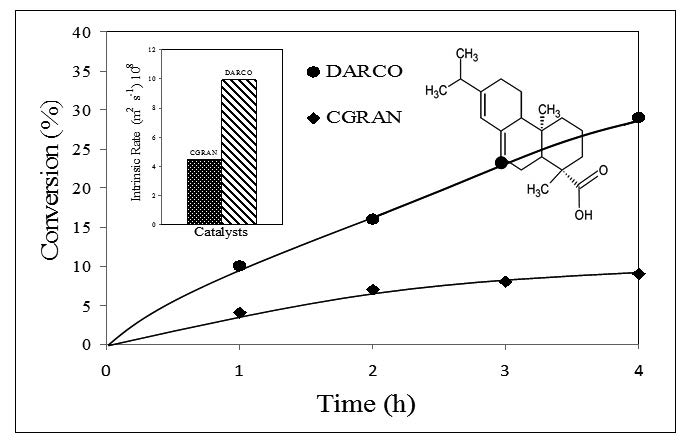
This study reports the adsorption and catalytic conversion of abietic acid as representative compound of tall oil, using activated carbons. Acid functional groups present on CGRAN activated carbons favored the adsorption of abietic acid, probably through a physical adsorption mechanism. In contrast, the conversion of abietic acid was not favored in DARCO activated carbon by increase of acid sites thought HNO3 treatment. The detection of neoabietic, palustric and/or levopimaric acids as reaction products indicate that the transformation of abietic acid was by dehydrogenation and/or isomerization routes. The negative influence of acid sites on the catalytic activity, in addition to the non-detection of volatile products, suggests that the cracking pathway for the conversion of abietic acid over these catalysts can be ruled out. Contrasting effects of the surface groups on the adsorption capacity and the conversion was observed: strong acid sites of CGRAN activated carbon favor the adsorption of abietic acid and decrease competitive adsorption between substrate and solvent, while conversion is not favored by these acid sites.
References
- Maggio G, and Cacciola G., Fuel98, 111–123, (2012).
- Sanches-Pereira A., Gómez M., J. Clean. Prod.,68, 1-15, (2014).
- Sharma R. K., Bakshi N. N., J. Chem. Eng.,69, 1071-1081, (1991).
- Demirbas A., Fuel, 90, 2273-2279, (2011).
- Coll R., Udas S., Jacoby W.A., Energy Fuels,15, 1166-1172, (2001).
- Clark I.T., Harris E.E., J. Am. Chem. Soc.,74, 1030-1032, (1952).
- Kang Y., Bhatia S.,Energy,35, 111-119, (2010).
- Junming X., Jianchun J., Jie Ch., YunjuanS.,Bioresour. Technol.,101, 5586–5591, (2010).
- Snare M, Kubickova I, Maeki-Arvela P, Eranen K, Murzin DY.,Ind. Eng. Chem Res., 45, 5708–5715, (2006).
- Wang L., Xiaopeng Ch., Jiezhen L., Yueyuan Ch., Xiaodong P., Tong T.,J. Chem. Eng., 152, 242-250, (2009).
- Haug P., Kraft A., ASM Science Journal,7, 67-68, (2013).
- Aguilar C., García R., Soto-Garrido G., Arriagada R., Top. Catal., 33, 201- 206, (2005).
- Cid R., Pecchi G., Appl. Catal.,14, 15-21, (1985).
- Poyet S., Charles S.,Cem. Concr. Res., 39, 1060 –1067, (2009).
- Liu J., Douglas M., Le Van, Carbon,48, 3454-3462, (2010).
- Wang G., GrathwohlP.,Environ. Pollut., 175, 110 – 116, (2013).
- El-Sharkawy I., Saha B., Koyama S., Srinivasan K,Int. J. Heat Mass Transfer,50, 902-907, (2007).
- Unger K., Angew. Chem. Int. Ed., 11, 267-278, (1972).
- Borislav D., Zdravkov, Čermák J.Jiří, ŠefaraMartin, JankůJosef, Cent. Eur. J. Chem., 5, 385-395, (2007).
- Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J., and Siemieniewska, T.,Pure Appl. Chem.,57, 603–619, (1985).
- Moreno-Castilla C., Lopez-Ramon M.V., Carrasco-MarınF.,Carbon, 38, 1995-2001, (2000).
- Figueiredo J., Pereira M., Freitas M., Orfao J., Carbon,37, 1379-1389, (1999).
- Zielke U., Hüttinger K.J., Hoffman W.P., Carbon, 34, 983-998, (1996).
- Swiatkowski A., Pakula M., Biniak S., Walczyk M., Carbon,42, 3057- 3069, (2004).
- Giles C., MacEwan T.H., Nakhwa S.N., Smith D., J. Chem. Soc., 3973-3993, (1960).
- Cabrita I., Ruiz B., Mestre A.S., Fonseca I.M., Carvalho A.P., AniaC.O.,Chem. Eng. J.,163, 249-255, (2010).
- Baccar R., Blánquez P., Bouzid J., Feki M., Attiya H., SarráM.,FuelProcess. Technol.,106, 408-415, (2013).
- Limousin G., Gaudet J.P., Charlet L., Szenknect S., Barthe`s V., Krimissa M., Appl. Geochem.,22, 249-275, (2007).
- Berger A.H., Bhown A. S., Energy Procedia,4, 562-567, (2011).
- Dell´mour M, Findeisen A, Kalm I, Baatz W and KenndlerE;The Open Analytical Chemistry Journal, 2, 67-73, (2008).
- Kersten P. J. , Kopper B. J. ,Raffa K. F. and Illman B. L.,J Chem Ecol.,32, 2679–2685, (2006).
- Souto J. C., Yustos P., Landeros M., García-Ochoa F., Bioresour. Technol., 102, 3504-3511, (2011).


