FOOT-OF-THE-WAVE ANALYSIS OF THE ELECTROCATALYTIC DECHLORINATION OF HEXACHLOROETHANE USING COBALOXIMES

- cobaloxime,
- cyclic voltammetry,
- organochlorines,
- catalysis
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
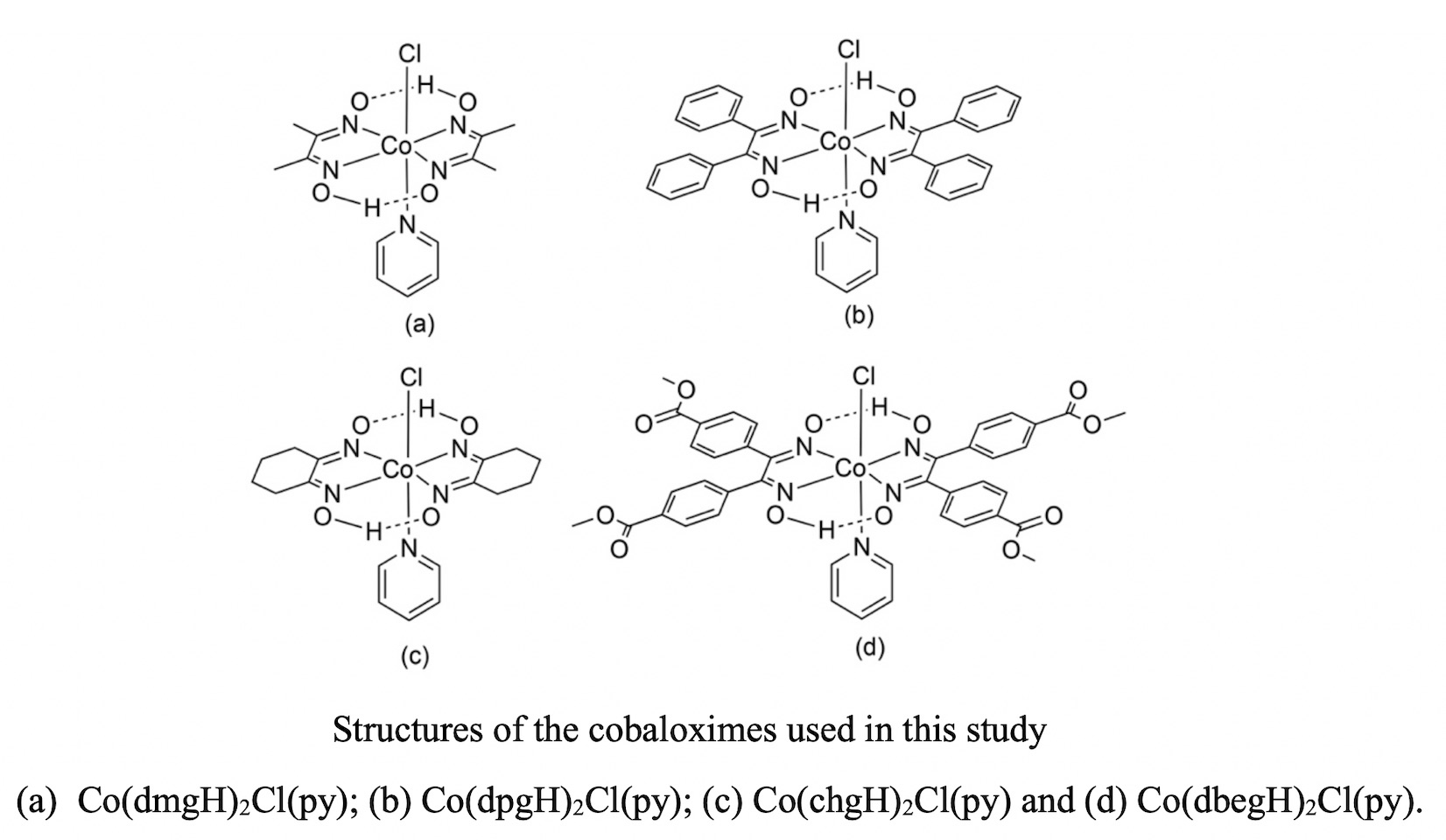
In this work the electrochemical degradation of polychlorinated compounds using Co(dmgH)2Cl(py), Co(dpgH)2Cl(py), Co(chgH)2Cl(py) and Co(dbegH)2Cl(py) (where dmgH is dimethylglyoximato, dpgH is diphenylglyoximato, chgH is 1,2-cyclohexanedionedioximato and dbegH is di(4-methylbenzoate)glyoximato) is described. The degradation was studied using cyclic voltammetry by monitoring current changes in the zone near to the Co(II/I) half wave potential as the concentration of the organochloride in the electrochemical cell is increased. Hexachloroethane (HCA) was used as organohalide substrate, while gamma-hexachlorocyclohexane (lindane), 1,2-dichloroethane, and 1,1,1-trichloroethane were used for comparative studies. The major dechlorination product of HCA, detected through head space GC-MS experiments after bulk electrolysis, was tetrachlorethylene. The rate constants of the dechlorination processes were estimated using the foot-of-the-wave analysis (FOWA), the values obtained were 1.10×105, 2.59×104, 4.91×104 and 1.83×104 for Co(dmgH)2Cl(py), Co(dpgH)2Cl(py), Co(chgH)2Cl(py) and Co(dpegH)2Cl(py) respectively.
References
- Pleština, R. PESTICIDES AND HERBICIDES | Types of Pesticide. in Encyclopedia of Food Sciences and Nutrition 90, 4473–4483 (Elsevier, 2003).
- Sparling, D. W. Organochlorine Pesticides. in Ecotoxicology Essentials 69–107 (Elsevier, 2016). doi:10.1016/B978-0-12-801947-4.00004-4
- Coakley, J., Bridgen, P., Bates, M. N., Douwes, J. & t Mannetje, A. Chlorinated persistent organic pollutants in serum of New Zealand adults, 2011–2013. Sci. Total Environ. 615, 624–631 (2018).
- Kim, S. A., Kim, K. S., Lee, Y. M., Jacobs, D. R. & Lee, D. H. Associations of organochlorine pesticides and polychlorinated biphenyls with total, cardiovascular, and cancer mortality in elders with differing fat mass. Environ. Res. 138, 1–7 (2015).
- Crinnion, W. J. Chlorinated pesticides: Threats to health and importance of detection. Altern. Med. Rev. 14, 347–359 (2009).
- Corsini, E., Liesivuori, J., Vergieva, T., Van Loveren, H. & Colosio, C. Effects of pesticide exposure on the human immune system. Hum. Exp. Toxicol. 27, 671–80 (2008).
- Dich, J., Zahm, S. H., Hanberg, A. & Adami, H. O. Pesticides and cancer. Cancer Causes Control 8, 420–443 (1997).
- Taiwo, A. M. A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere 220, 1126–1140 (2019).
- Zhu, W. et al. A New Strategy towards Efficient and Recyclable Carbon-Chloride Bond Cleavage of Environmentally Harmful Organochlorides through Electrochemical Catalysis in Non–aqueous Media. ChemistrySelect 2, 645–649 (2017).
- Fritsch, J. M. & McNeill, K. Aqueous reductive dechlorination of chlorinated ethylenes with tetrakis(4-carboxyphenyl)porphyrin cobalt. Inorg. Chem. 44, 4852–4861 (2005).
- WHO. IARC MONOGRAPHS ON THE EVALUATION OF CARCINOGENIC RISKS TO HUMANS. IARC, International Agency for Research on Cancer 79, (2001).
- Aulenta, F., Majone, M., Verbo, P. & Tandoi, V. Complete dechlorination of tetrachloroethene to ethene in presence of methanogenesis and acetogenesis by an anaerobic sediment microcosm. Biodegradation 13, 411–424 (2002).
- Ma, C. & Wu, Y. Dechlorination of perchloroethylene using zero-valent metal and microbial community. Environ. Geol. 55, 47–54 (2008).
- Kliegman, S. & McNeill, K. Dechlorination of chloroethylenes by cob(I)alamin and cobalamin model complexes. Dalt. Trans. 9226, 4191–4201 (2008).
- Costentin, C., Robert, M. & Savéant, J. M. Successive removal of chloride ions from organic polychloride pollutants. Mechanisms of reductive electrochemical elimination in aliphatic gem-polychlorides, α,β-polychloroalkenes, and α,β -polychloroalkanes in mildly protic medium. J. Am. Chem. Soc. 125, 10729–10739 (2003).
- Assaf-Anid, N., Hayes, K. F. & Vogel, T. M. Reduction dechlorination of carbon tetrachloride by cobalamin(II) in the presence of dithiothreitol: mechanistic study, effect of redox potential and pH. Environ. Sci. Technol. 28, 246–252 (1994).
- Rich, A. E., DeGreeff, A. D. & McNeill, K. Synthesis of (chlorovinyl)cobaloxime complexes, model complexes of proposed intermediates in the B12-catalyzed dehalogenation of chlorinated ethylenes. Chem. Commun. 2, 234–235 (2002).
- Bhattacharjee, A. et al. Combined Experimental–Theoretical Characterization of the Hydrido-Cobaloxime [HCo(dmgH) 2 (P n Bu 3 )]. Inorg. Chem. 51, 7087–7093 (2012).
- Kaeffer, N., Chavarot-Kerlidou, M. & Artero, V. Hydrogen evolution catalyzed by cobalt diimine-dioxime complexes. Acc. Chem. Res. 48, 1286–1295 (2015).
- Valdez, C. N., Dempsey, J. L., Brunschwig, B. S., Winkler, J. R. & Gray, H. B. Catalytic hydrogen evolution from a covalently linked dicobaloxime. Proc. Natl. Acad. Sci. 109, 15589–15593 (2012).
- Lawrence, M. A. W. et al. Computational, electrochemical, and spectroscopic studies of two mononuclear cobaloximes: the influence of an axial pyridine and solvent on the redox behaviour and evidence for pyridine coordination to cobalt( i ) and cobalt( ii ) me. Dalt. Trans. 45, 10326–10342 (2016).
- Losse, S., Vos, J. G. & Rau, S. Catalytic hydrogen production at cobalt centres. Coord. Chem. Rev. 254, 2492–2504 (2010).
- Pizarro, S., Araya, M. & Delgadillo, A. Hexachloroethane reduction catalyzed by cobaloximes. Effect of the substituents on the equatorial ligands. Polyhedron 141, 94–99 (2018).
- Fan, W. Y., Tan, Z. Bin & Koh, J. I. Proton reduction using cobalt glyoximes with isothiocyanate and aniline axial ligands. Polyhedron 96, 38–43 (2015).
- Concepción, S., Aguiló, M., Solans, X. & Font-Altaba, M. Synthesis and Structure of Chloro ( ligand ) bis ( diphenylglyoximato ) cobalt ( Complexes. Inorganica Chim. Acta 127, 153–159 (1987).
- Xin, Z., Deyan, H., Yizhi, L. & Huilan, C. Structure and thermal decomposition studies on alkylcobaloxime B 12 model compounds with 1,2-cyclohexanedione dioxime as equatorial ligand. Inorganica Chim. Acta 359, 1121–1128 (2006).
- Pizarro, S., Gallardo, M., Gajardo, F. & Delgadillo, A. Electrochemical reduction of lindane using a cobaloxime containing electron-withdrawing groups. Inorg. Chem. Commun. 99, 164–166 (2019).
- Panagiotopoulos, A., Ladomenou, K., Sun, D., Artero, V. & Coutsolelos, A. G. Photochemical hydrogen production and cobaloximes: the influence of the cobalt axial N-ligand on the system stability. Dalt. Trans. 45, 6732–6738 (2016).
- Lee, K. J., Elgrishi, N., Kandemir, B. & Dempsey, J. L. Electrochemical and spectroscopic methods for evaluating molecular electrocatalysts. Nat. Rev. Chem. 1, 0039 (2017).
- Costentin, C., Drouet, S., Robert, M. & Savéant, J. M. Turnover numbers, turnover frequencies, and overpotential in molecular catalysis of electrochemical reactions. Cyclic voltammetry and preparative-scale electrolysis. J. Am. Chem. Soc. 134, 11235–11242 (2012).
- Costentin, C. & Savéant, J.-M. Multielectron, Multistep Molecular Catalysis of Electrochemical Reactions: Benchmarking of Homogeneous Catalysts. ChemElectroChem 1, 1226–1236 (2014).
- Costentin, C. & Savéant, J.-M. Multielectron, Multistep Molecular Catalysis of Electrochemical Reactions: Benchmarking of Homogeneous Catalysts. ChemElectroChem 1, 1226–1236 (2014).
- Elgrishi, N., Chambers, M. B. & Fontecave, M. Turning it off! Disfavouring hydrogen evolution to enhance selectivity for CO production during homogeneous CO 2 reduction by cobalt–terpyridine complexes. Chem. Sci. 6, 2522–2531 (2015).
- Costentin, C. & Savéant, J. M. Homogeneous Molecular Catalysis of Electrochemical Reactions: Catalyst Benchmarking and Optimization Strategies. J. Am. Chem. Soc. 139, 8245–8250 (2017).

