- Adsorption,
- Denitrogenation,
- Nickel,
- Pyridine
Copyright (c) 2017 Cecilia Peralta, Esteban Camú, R. Bassi, Mirza Villarroel, Juan Ojeda, Patricio Baeza

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
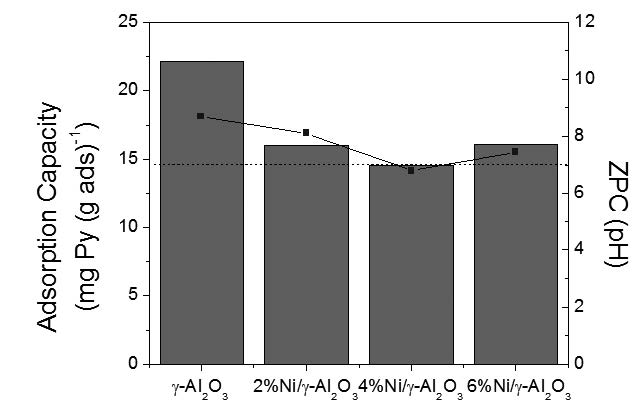
In this work we have carried out the adsorption of pyridine using three different supports (activated carbon, SiO2 and γ-Al2O3). After choosing the best support, due to its higher adsorption capacity, we have impregnated the support with nickel at three different concentrations (2, 4 and 6% w/w) by wet impregnation to study the adsorption of pyridine by π-complexation. All the samples (supports and adsorbents) were characterized by N2 adsorption-desorption by the BET method and electrophoretic migration. The experimental results, for the three different supports, show that the adsorption capacity is better for γ-Al2O3, due to its higher isoelectric point. With the incorporation of nickel, no better adsorption capacities are observed, due that the nickel incorporation diminish the zero point charges of the adsorbents.
References
- J.B. Speight, Chemistry and Technology of Petroleum, New York (1998).
- S. Shin, K. Sakanishi, I. Mochida, D.A. Grudoski, J.H. Shinn. Energy & Fuels 14, 539-544 (2000).
- G.C. Laredo, S. Leyva, R. Alvarez, M.T. Mares, J. Castillo, J.L. Cano, Fuel 81, 1341-1350 (2002).
- P.Grange, Catal. Rev.-Sci.Eng. 21, 135-181 (1980).
- A.K. Mathur, C.B. Majumder, S. Chatterjee, J. Hazard. Mater. 157, 335- 343 (2008).
- K.V. Padoley, A.S. Rajvaidya, T.V. Subbarao, Biores. Technol. 97, 1225- 1236 (2006).
- L. Qiao, J. Wang, J. Hazard. Mater. 176, 220-225 (2010).
- H. Zhao, S. Xu, J. Zhong, X. Bao, Catal. Today 93-95, 857-861 (2004).
- R. Andreozzi, A. Insola, V. Caprio, M.G. D’Amore, Water Res. 25, 655- 659 (1991).
- M.K. Mandal, P.K. Bhattacharya, J. Membrane Sci. 286, 115-124 (2006).
- M.E. Essington, Environ. Geol. Water Sci. 19, 83-89 (1992).
- D.H. Lataye, I.M. Mishra, I.D. Mall, J. Hazard. Mater. 154, 858-870 (2008).
- D. Mohan, K.P. Singh, S. Sinha, D. Gosh, Carbon 43, 1680-1693 (2005).
- J. Ren, J. Wang, C. Huo, X. Wen, Z. Cao, S. Yuan, Surf. Sci. 601, 1599- 1607 (2007).
- P. Baeza, G. Aguila, F. Gracia, P. Araya, Catal. Commun. 9, 751-755 (2008).
- P. Baeza, G. Aguila, G. Vargas, J. Ojeda, P. Araya, Appl. Catal. B 111,133- 140 (2012).
- A.J.Hernandez-Maldonado, R.T. Yang, AIChE Journal 50(4), 791-801 (2004).
- F. Aparicio, E. Camú, M. Villarroel, N. Escalona, P. Baeza, J. Chil. Chem. Soc. 58, 2057-2060 (2013).
- O. Hamdaoui, E. Naffrechoux. J. Hazard Mater. 147, 381–394 (2007).
- A.K. Bajpai, M. Rajpoot, D.D. Mishra, J. Colloid Interface Sci. 187, 96– 104 (1997).
- C. Huang, J. Colloid Interface Sci. 53,178–186 (1975).
- E. Baumgarten, U. Kirchhausen-Dusing, J. Colloid Interface Sci. 194, 1–9 (1997).
- T. Tranior, G. Brown Jr., G. Parks, J. Colloid Interface Sci. 231, 359–372 (2000).
- J. Kwon, J. Moon, Y. Bae, D. Lee, H. Sohn, C. Lee. ChemSusChem. 1, 307 – 309 (2008).
- A. Koriakin, K. Muruganandam, C. Lee. Chem. Eng. J. 162, 649-655 (2010).
- J.-P. Bellat, I. Bezverkhyy, G. Weber, S. Royer, R. Averlant, J.-M. Giraudon and J.-F. Lamonier, J. Hazard. Mater. 300 711-717 (2015).
- H. Yamada, D. S. Dao, J. Fujiki and K. Yogo, Sep. Sci. Technol. 50, 2948- 2953 (2015) Department of Chemical and Biomolecular Engineering, Yonsei University, 262 SeongSanno, Seodaemun-gu, Seoul 120-749, South Korea Received 6 April 2010, Revised 9 June 2010, Accepted 9 June 2010, Available online 17 June 2010.
- F.J. Gil Llambias, A.M. Escudey, J. Santos-Blanco, J. Catal. 83, 225-228 (1983).


