INTRODUCING AN EFFECTIVE NANOCATALYTIC FOR THE ONE-POT SYNTHESIS AND INVESTIGATION OF BIOLOGICAL PROPERTIES OF PYRANOPYRIMIDINONE AND XANTHENE DERIVATIVES

- zinc oxide-chitosan nanocomposite,
- microwave,
- pyranopyrimidinone,
- xanthene,
- Biological activity
- MIC,
- MBC. ...More
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
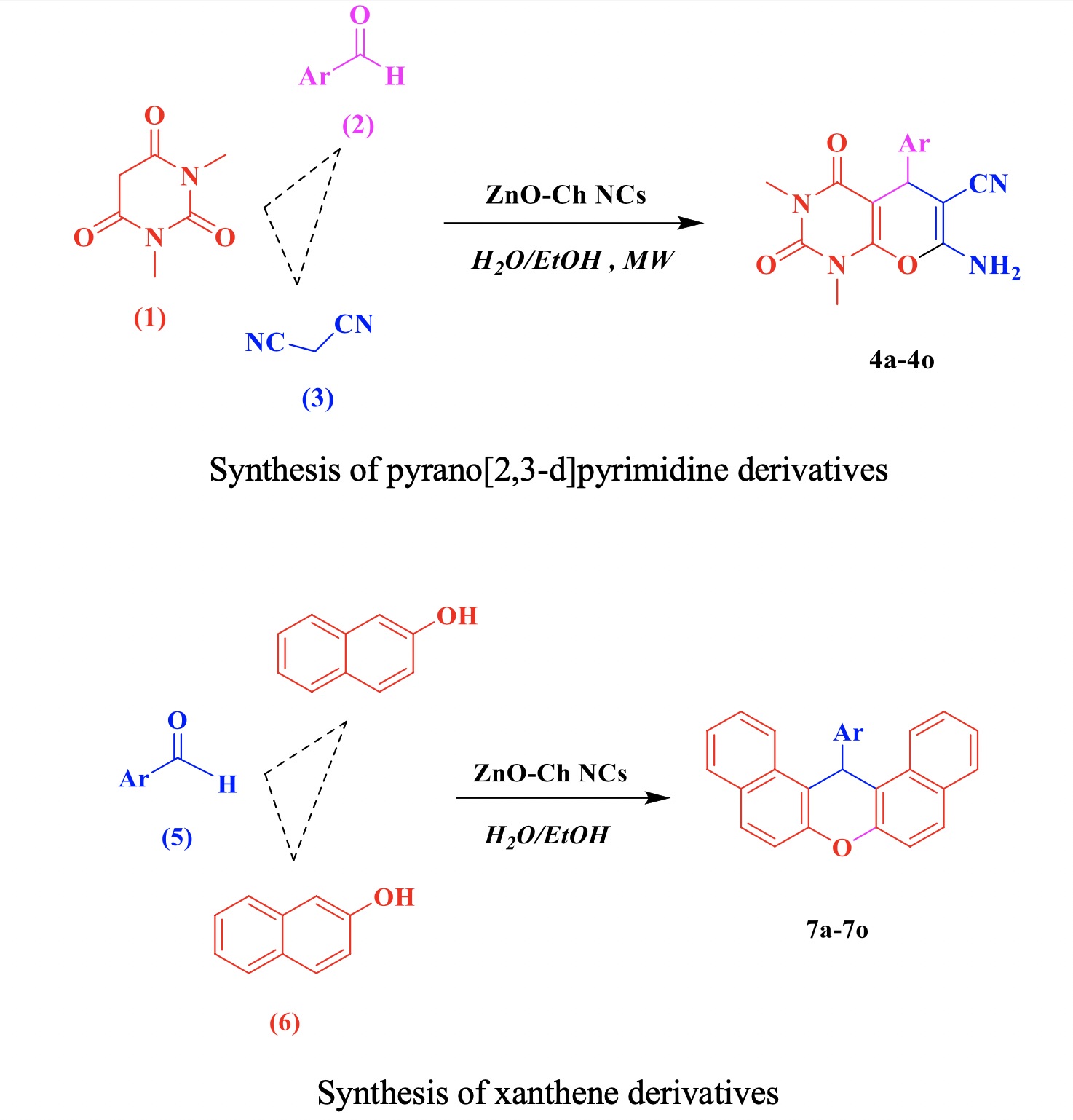
In this study, an effective method for the synthesis of pyranopyrimidinone and xanthene derivatives is introduced. In this method, zinc oxide-chitosan nanocomposite has been used as a catalyst and all reactions were performed under irradiated in a microwave oven at a power of 230W. The properties of zinc oxide-chitosan nanocomposite were characterized by X-ray diffraction (XRD), energy dispersive X-ray (EDAX) analysis and scanning electron microscopy (SEM). The results indicated that the size of zinc oxide-chitosan nanocomposite varies between 76–95 nm. The catalyst activity has maintained its activity after four runs. The main advantages of this method are; Eco-friendly procedure, catalytic synthesis of affordable and inexpensive materials, short reaction time, and excellent yields which makes it more economic than the other conventional methods. The antibacterial activity of the products was investigated using Minimum Inhibitory Concentration (MIC) and the Minimum Bactericidal Concentration (MBC) methods. Pyranopyrimidinone derivatives exhibit more antibacterial activity.
References
- References
- A. K. Bhattachary, K. C. Rana, Mendeleev Commun. 17, 247, (2007).
- M. T. Maghsoodlou, R. Heydari, M. Lashkari, F. Mohamadpour, Indian J Chem. 56B, 160, (2017).
- M. Seyyedhamzeh, M. Mirzaei, A. Bazgir, Dyes Pigm. 76, 836, (2008).
- R. Gupta, A. Jain, R. Joshi, M. Jain, Bull Korean Chem Soc. 32, 899, (2011).
- M. M. Hanna, Eur J Med Chem. 55, 12, (2012).
- S. V. Shinde, W. N. Jadhav, N. N. Karade, Orient J Chem. 26, 307, (2010).
- S. Gupta, P. Gupta, A. Sachar, R. L. Sharma, Indian J Chem, 49, 1243, (2010).
- Z. Karimi-Jaberi, M. M. Hashemi, Monatsh Chem. 139, 605, (2008).
- W. Su, D. Yang, C. Jin, B. Zhang, Tetrahedron Lett. 49, 3391, (2008).
- J. Q. Wang, R. G. Harvey, Tetrahedron. 58, 5927, (2002).
- A. Jha, J. Beal, Tetrahedron Lett. 45, 8999, (2004).
- R. J. Sarma, J. B. Baruah, Dyes Pigm. 64, 91, (2005).
- S. Kobayashi, T. Busujima, S. Nagayama, Chem Eur J. 6, 3491, ( 2000)
- M. G. Constantino, V. Lacerda, Synth. Commun. 37, 3529, (2007).
- C. K. Z. Andrade, Curr Org Synth. 1, 333, (2004).
- P. Kulkarni, J Mex Chem Soc. 62, 1, (2018).
- K. Gong, D. Fang, H. L. Wang, X. L. Zhou, Z. L. Liu, Dyes Pigm. 80, 30, (2009).
- D. Liu, S. Zhou, J. Gao, L. Li, D. Xu, J Mex Chem Soc. 57, 345, (2013).
- A. Rahmatpour, Monatsh Chem. 142, 1259, (2011).
- R. Kumar, G. C. Nandi, R. K. Verma, M. S. Singh, Tetrahedron Lett. 51, 442, (2010).
- J. Q. Wang, R. G. Harvey, Tetrahedron. 58, 5927, (2002)
- H. Stetler, W. Forest, Academic Press NY. 2, 51, (1963).
- S. Ko, C. F. Yao, Tetrahedron Lett. 47, 8827, (2006).
- K. Gong, D. Fang, H. L. Wang, X. L. Zhou, Z. L. Liu, Dyes Pigm. 80, 30, (2009).
- M. H. Majid, A. Hamideh, B. Khadijeh, S. Mina, A. O. Hossein, F. B. Fatemeh, Bull Chem Soc Ethiop. 25, 399, (2011).
- B. L. Hayes, Microwave Synthesis Chemistry at the Speed of Light, CEM Publishing, 2002.
- N. E. Leadbeater, Microwave Heating as a Tool for Sustainable Chemistry, CRC Press, 2010.
- J. P. Tierney, P. Lidstroem, Microwave Assisted Organic Synthesis. CRC Press, 2005.
- C. O. Kappe, A. Stadler, D. Dallinger, Microwave in Organic and Medicinal Chemistry. Wiley-VCH-Verlag, 2012.
- F. Smith, W. Kenneth, A. Humera, B. Lorraine, L. Laberge, J. Rousell, Tetrahedron Lett. 27, 279, (1986).
- L. Jin, Y. Zhang, L. Yan, Y. Guo, L. Niu, Molecules. 17, 9361, (2012).
- S. A. H. Vahabi, F. Hatamjafari, K. Pourshamsian, O J C. 30, 849, (2014).
- N. Montazeri, K. Pourshamsian, H. rezaei, M. Fouladi, Asian J Chem. 25, 3463, (2013).
- N. Montazeri, K. Pourshamsian, M. Bayazi, S. Kabiri, Asian J Chem. 25, 3373, (2013).
- N. Montazeri, K. Pourshamsian, M. Rahgol, M. Bayazi, Asian J Chem. 24, 5361, (2012).
- N. Montazeri, K. Pourshamsian, A. Fomani, S. J. Kalantarian, Asian J Chem. 24, 2805, (2012).
- K. Pourshamsian, N. Montazeri, K. Rad-Moghadam, S. A. Asgari, J Heterocycl Chem. 47, 1439, (2010).
- M. A. Pasha, M. Krishnappa, V. P. Jayashankara, Indian J Chem. 49B, 1428, (2010).
- V. K. Alhuwalia, R. A, Batla, Khurana, R. Kumar, Indian J Chem Sec-B. 29, 1141, (1990).
- M. Jinxia, Z. Wenhua, T. Yajun, W. Zhiguo, Nanoscale Res Lett. 11, 1, (2016).
- J. Albadi, A. Mansournezhad, T. Sadeghi, Res Chem Intermed. 41, 8317, (2015).
- B. Sabour, M. H. Peyrovi, M. Hajimohammadi,, Res Chem Intermed. 41, 1343, (2015).
- N. Sheikhan-Shamsabadi, M. Ghashang, Main Group Metal Chemistry. 40, 19, (2017).
- A. R. Bhat, A. H. Shalla, R. S. Dongre, J. Taibah, J Univ Sci. 10, 9, (2016).
- A. Mobinikhaledi, M. A. Bodaghi, Acta Chim. Slov. 57, 931, (2010).
- H. Kefayati, M. Valizadeh, A. Islamnezhad, Anal Bioanal Electrochem. 6, 80, (2014).
- G. H. Mahdavinia, S. Rostamizadeh, A. M. Amani, Z. Emdadi, Ultrason Sonochem. 16, 7, (2009).
- K. Gong, D. Fang, H. L. Wang, X. L. Zhou, Z. L. Liu, Dyes Pigm. 80, 30, (2009).
- B. B. F. Mirjalili, A. Bamoniri, A. Akbari, Tetrahedron Lett. 49, 6454, (2008).
- P. Kumari, V. Yathindranath, M. S. Chauhan, Synth. Commun. 38, 637, (2008).
- M. A. Pasha, V. P. Jayashankara, Bioorg Med. Chem. Lett. 17, 621, (2007).
- J .V. Madhav, B. S. Kuarm, B. Rajitha, ARKIVOC. 2, 204, (2008).
- F.Q. Ding, L.T. An, J. P. Zou, Chinese J Chem. 25, 645, (2007).
- J. P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf, R. Lacroix, Eur. J. Med. Chem. 13, 67, (1978).
- S. Rostamizadeh, N. Shadjou, A.M. Amani, S. Balalaie, Chinese Chem Lett, 19, 1151, (2008).
- M.M. AbdElhady, J Carbohyd Chem. 2012, 1, (2012).

