DETERMINATION OF TRICLABENDAZOLE IN CATTLE PLASMA AS ITS SULPHOXIDE AND SULPHONE METABOLITES BY ROTATING DISK SORPTIVE EXTRACTION COMBINED WITH HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY AND ITS APPLICATION TO PHARMACOKINETIC STUDIES
- Triclabendazole,
- Triclabendazole sulfoxide,
- Triclabendazole sulfone,
- Cattle plasma,
- Rotating disk sorptive extraction
- HPLC ...More
Copyright (c) 2017 Alejandro Cañas-Mülle, Marcial Vargas del Campo, Pablo Richter

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
In this study, a new method for determination of triclabendazole (TCB) as its main metabolites, triclabendazole sulfoxide (TCB-SO) and triclabendazole sulfone (TCB-SO2) in animal plasma was developed. TCB is widely used as antiparasitic in the veterinary industry. Rotating disk sorptive extraction (RDSE) was the selected sample preparation technique for extraction/clean up of the compounds from the samples followed by high performance liquid chromatography coupled to diode array detection (HPLC-DAD) for quantification.
Optimization of physicochemical variables was performed using a multivariate experimental design. Following the recommendations given in the Veterinary International Conference on Harmonization guides (VICH GL02 and VICH GL49), the validation of the method was performed, given rise to improved analytical features compared with those provided by other methods based on solid phase extraction (SPE). Linearity (r > 0.99) was achieved for both compounds when calibration was performed not only in standard solutions but also in animal spiked plasma. Selectivity, defined as the response ratio between blank and analyte at the limit of quantification, was 11.8% and 3.4% for TCB-SO and TCB-SO2, respectively. Accuracy and precision, expressed in percentage, were always lower than -16.7% and 8.1%, respectively. Eco-efficiency was quantitatively assessed indicating that the method is an excellent green method.
The proposed analytical method was applied to the determination of the pharmacokinetic of two commercial products of TCB in cattle plasma after oral administration.
The good analytical performance, eco-efficiency and economy, make the method interesting for alternative use in routine laboratories.
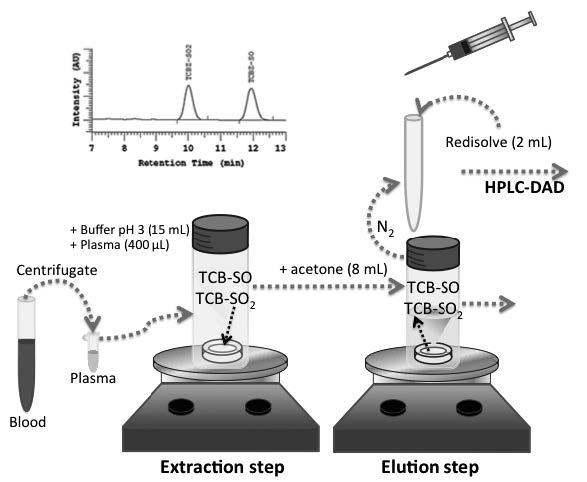
References
- Bennett J., Köhler P. Exp. Parasitol. 63, 49, (1987).
- Hennessy D.R., Lacey E., Steeel J.W., Prichard R.K. J. Vet. Pharmacol. Ther. 10, 64, (1987).
- Virkel G., Lifschitz A., Sallovitz J., Pis A., Lanusse C. J. Vet. Pharmacol. Ther. 29, 213, (2006).
- Arnorld D. (2012) Addendum to the monographs prepared by the 40th, 66th and 70th Meetings of the Committee and published in FAO Food & Nutrition Paper 41/5 & FAO JECFA Monographs 2 and 6, respectively. 1–75, Webpage visited at 10th May 2016. ftp://ftp.fao.org/ag/agn/jecfa/ vetdrug/12-2012-triclabendazole.pdf
- Bull M.S., Shume G.R.E. J. Pharm. Biomed. Anal. 5, 527, (1987).
- Sanyal P.K. Ind. J. Pharmacol. 26, 200, (1994).
- Formentini E.A., Mestorino N., Pesoa J.M., Lucas M., Reggiardo E., Marti-Diaz M., Reutemann S.H., Errecalde J.O. Rev. FAVE – Cienc. Vet. 3, 39, (2004).
- Mestorino N., Formentini E.A., Lucas M.F., Fernandez C., Modamio P., Mariño-Hernández E., Errecalde J.O. Vet. Res. Commun. 32, 21, (2008).
- Cai Ch., Xue F., Wang Z., Xiao S., Zhang L. Food Anal. Methods 5, 1260, (2012).
- Cai Ch., Zhang L., Xue F., Qiu M., Zheng W. J. Chromatogr. B 878, 3106, (2010).
- Belal F., El-Din M.K.Sh., Elenany N., Saad S. Luminescence 29, 559, (2014).
- Zrnčić M., Gros M., Babić S., Kaštelan-Macan M., Barcelo D., Petrović M. Chemosphere 99, 224, (2014).
- Cañas A., Valdebenito S., Richter P. Anal. Bioanal. Chem. 406, 2205, (2014).
- Manzo V., Honda L., Navarro O., Ascar L., Richter P. Talanta 128, 486, (2014).
- Validation of Analytical Procedures: Methodology: Final Guidance VICH GL2 (1998) 1–10 Webpage visited at 10th May 2016. http://www.fda. gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/ GuidanceforIndustry/UCM052379.pdf
- Validation of Analytical Methods Used in Residue Depletion Studies VICH GL49 (2015) 1–23. Webpage visited at 10th May 2016. http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/ GuidanceforIndustry/UCM207942.pdf
- Good Clinical Practice. VICH GL9 (2000) 1–31. Webpage visited at 10th May 2016. http://www.fda.gov/downloads/AnimalVeterinary/ GuidanceComplianceEnforcement/GuidanceforIndustry/UCM052417.pdf
- Richter P., Cañas A., Muñoz C., Leiva C., Ahumada I. Anal. Chim. Acta. 695, 73, (2011).
- Jachero L., Sepúlveda B., Ahumada I., Fuentes E., Richter P. Anal. Bioanal. Chem. 405, 7711, (2013).
- Blahová E., Brandšteterová E. Chem. Pap. 58, 362, (2004).
- Gałuszka A., Konieczka P., Migaszewski Z.M., Namiesnik J. Trends Anal. Chem. 37, 61, (2012).


