ANTIPROLIFERATIVE ACTIVITY OF NEW 6-BROMINE DERIVATIVES OF 7-ANILINO-1- ARYLISOQUINOLINEQUINONES
- Isoquinolinequinones,
- Half-wave potentials,
- MTT assay,
- Antiproliferative activity
Copyright (c) 2017 Juana Andrea Ibacache, Jaime A. Valderrama, Verónica Arancibia, Cristina Theoduloz, Giulio G. Muccioli, Julio Benites

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
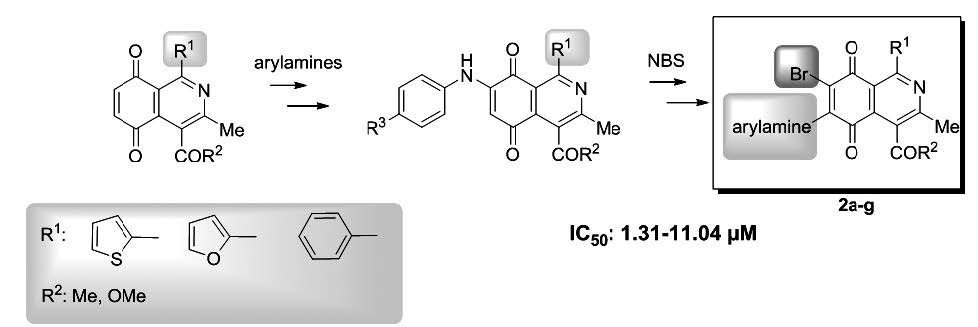
A variety of 6-bromine-containing 7-anilino-1-arylisoquinolinequinones 2a-g were synthesized to evaluate their half-wave potentials and in vitro antiproliferative activity on gastric and leukemia cancer cell lines. The new compounds displayed significant IC50 values in the range: 1.31 to 11.04 μM. The structure activity relationship analysis of the new series suggest that the antiproliferative activity is dependent, in part, on the push-pull electronic effects of the nitrogen and bromine substituents inserted into the redox fragment of the 1-arylisoquinolinequinone scaffold. Linear regression analysis provided satisfactory relationships between the log IC50 and ClogP values for the AGS gastric cancer cell line.
References
- W. Hai-Quian, H. Zhi-Shu, B. Xian-Zhang, S. Yu-Dong, Z. Zhu-Lin, X. Bing-Fen, L. Zong-Chao, G. Lian-Quan, C. Albert. Eur. J. Med. Chem. 40, (2005), 1341–1345.
- L. Rossi, G.A. Moore, S. Orrenius, P.J. O’Brien. Arch. Biochem. Biophys. 251, (1986), 25–35.
- M.A. Tapper, B.R. Sheedy, D.E Hammermeister, P.K. Schmieder. Toxicol. Sci. 55 (2000), 327–334.
- R. Osman, K. Namboodiri, H. Weinstein, J.R. Rabinowitz. J. Am. Chem. Soc. 110, (1988), 1701–1707.
- A. Plubrukran, S. Yuenyongsawad, T. Thammasaroj, A. Jitsue. Pharm. Biol. 41, (2003), 439.
- T. Sandoval, R.A. Davis, T.S. Bugni, G.P. Concepcion, M.K. Harper, C.M. Ireland. Nat.Prod.Res. 18, (2004), 89.
- U.W. Hawas, M. Shaaban, K.A. Shaaban, M. Speitling, A. Maier, G. Kelter, H.H. Fiebig, M. Meiners, E. Helmke, H. Laatsch. J. Nat. Prod. 71, (2009), 2120–2124.
- D.J. Milanowski, K.R. Gustafson, J.A. Kelley, J.B. McMahon. J. Nat. Prod. 67, (2004), 70–73.
- P. Wipf, B. Joo, T. Nguyenb, J.S. Lazo. Org. Biomol. Chem. 2, (2004), 2173–2174.
- M. Brisson, C. Foster, P. Wipf, B. Joo, R.J. Tomk, T. Nguyen, J.S. Lazo. Mol. Pharmacol. 71, (2007), 184–192.
- J.A. Valderrama, J.A. Ibacache, V. Arancibia, J. Rodriguez, C. Theoduloz. Bioorg. Med. Chem. 17, (2009), 2894–2901.
- V. Delgado, J.A. Ibacache, C. Theoduloz, J.A. Valderrama. Molecules. 17, (2012), 7042–7056.
- V. Delgado, J.A. Ibacache, V. Arancibia, C. Theoduloz, J.A. Valderrama. Molecules. 18, (2013), 721–734.
- J.S. Lazo, D.C. Aslan, E.C. Southwick, K.A. Cooley, A.P. Ducruet, B. Joo, A. Vogt, P. Wipf. J. Med. Chem. 44, (2001), 4042–4049.
- B.J. Mulchin, C.G. Newton, J.W. Baty, C.H. Grasso, W.J. Martin, M.C. Walton, E.M. Dangerfield, C.H. Plunkett, M.V. Berridge, J.L. Harper, J.L. Bioorg. Med. Chem. 18, (2010), 3238–3251.
- V.K. Tandom, H.K. Maurya, N.N. Mishra. Eur. J. Med. Chem. 44, (2009), 3130–3137.
- J.A. Ibacache, V. Delgado, J. Benites, C. Theoduloz, V. Arancibia, G. Muccioli, J.A. Valderrama. Molecules. 19, (2014), 726-739.
- Y. Prieto, M. Muñoz, V. Arancibia, M. Valderrama, F.J. Lahoz, M.L. Martín. Polyhedron. 26, (2007), 5527–5532.
- F.C. De Abreu, P.A. de Ferraz, M.O.F. Goulart. J. Braz. Chem. Soc.13, (2002), 19–35.
- M. Aguilar-Martinez, G. Cuevas, M. Jimenez-Estrada, I. González, B. Lotina-Hennsen, N. Macias-Ruvalcaba. J. Org. Chem. 64, (1999), 3684– 3694.
- M.C. Alley, D.A. Scudiero, A. Monks, M.L. Hursey, M.J. Czerwinski, D.L. Fine, B.J. Abbott, J.G. Mayo, R.H. Shoemaker, M.R. Boy. Cancer Res. 48, (1988), 589–601.
- A.A.Van de Loosdrecht, R.H. Beelen, G.J. Ossenkoppele, M.G. Broekhoven, M.M. Langenhuijsen. J. Immunol. Methods. 174, (1994), 311–320.
- D.A. Scudiero, R.H. Shoemaker, K.D. Paull, A. Monks, S. Tierney, T.H. Nofziger, M.J. Currens, D. Seniff, M.R. Boyd. Cancer Res.48, (1988), 4827–4833.
- E. M. Hodnett, C. Wongwiechintana, W.J. Dunn, P.J. Marra. J. Med. Chem. 26, (1983), 570.
- R.P. Verma. Anti-Cancer Agent Med. Chem. 6, (2006), 489.


