- Finex AS510GC,
- Ion exchange,
- Isophthalic acid,
- Phthalic acid,
- Regeneration
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
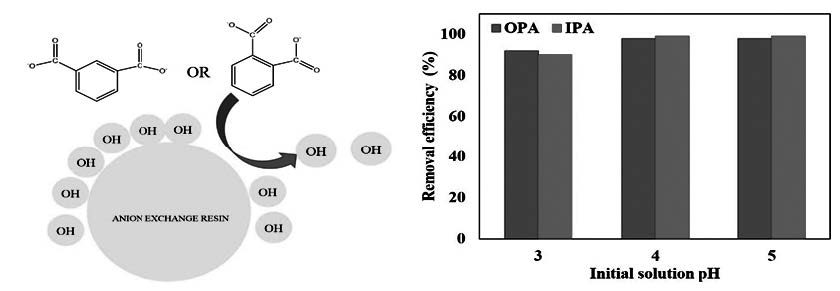
This study investigated the efficiency of a strongly basic anion exchange resin for the removal of phthalic acid (also known as orto-phthalic acid) and isophthalic acid in aqueous solutions. Uptake behavior of resin towards phthalic acid and isophthalic acid has been assessed as a function of resin dose, initial solution pH, and temperature. The pseudo-second-order rate equation fitted well kinetic profiles for such sorbates. Phthalic acid and isophthalic acid loading isotherm studies have been performed and experimental data were analyzed by Freundlich, Langmuir and Dubinin–Radusckevich (D-R) equations. The maximum sorption capacity of the resin predicted by the Langmuir model were 397.8 mg g−1 for the phthalic acid and 331.3 mg g−1 for isophthalic acid. The mean free sorption energies through the D-R model suggest that sorption takes place mainly through an ion exchange mechanism. The experimental results illustrated that the sorption of phthalic acid onto anion exchange resin is feasible, spontaneous and exothermic however, sorption of isophthalic acid is endothermic. The successful phthalic acid-loaded resin regeneration in a batch system revealed the higher efficiency of the 2.0 M HCl solution compared to 1.0 M HCl and 1.0 M NaOH as desorbing agents. In the case of isophthalic acid-loaded resin, 2.0 M NaCl solution found to be most efficient regenerant.
References
- K. M. Gani and A. A. Kazmi, Crit. Rev. Environ. Sci. Technol. 46, 1402 (2016).
- M. Julinova and R. Slavik, J. Environ. Manage. 94, 13 (2012).
- W. Zhang, Z. Xu, B. Pan, L. Lv, Q. Zhang, Q. Zhang, W. Du, B. Pan, and Q. Zhang, J. Colloid Interface Sci. 311, 382 (2007).
- M. M. Abdel daiem, J. Rivera-Utrilla, R. Ocampo-Pérez, J. D. Méndez- Díaz, and M. Sánchez-Polo, J. Environ. Manage. 109, 164 (2012).
- M. Matsumoto, M. Hirata-Koizumi, and M. Ema, Regul. Toxicol. Pharmacol. 50, 37 (2008).
- Y. Wang, G. Zhang, and L. Wang, J. Agric. Food Chem. 63, 75 (2015).
- A. Gómez-Hens and M. P. Aguilar-Caballos, TrAC - Trends Anal. Chem. 22, 847 (2003).
- D. W. Gao and Z. D. Wen, Sci. Total Environ. 541, 986 (2016).
- N. A. Khan, B. K. Jung, Z. Hasan, and S. H. Jhung, J. Hazard. Mater. 282, 194 (2015).
- E. Parlak and Ö. Arar, J. Dispers. Sci. Technol. 39, 1403 (2018).
- C.-M. Park and R. J. Sheehan, in Kirk-Othmer Encycl. Chem. Technol. (2000), pp. 17–18.
- C. Kaya, A. Şahbaz, Ö. Arar, Ü. Yüksel, and M. Yüksel, Desalin. Water Treat. 55, (2015).
- M. A. Güngör, Ö. Özalp, and Ö. Arar, Desalin. Water Treat. 88, 279 (2017).
- B. Alyüz and S. Veli, J. Hazard. Mater. 167, 482 (2009).
- P. Liu, Y. Li, Y. Xu, Y. Qing, and C. Han, J. Chil. Chem. Soc. 63, 3819 (2018).
- A. Erdem Yayayürük and O. Yayayürük, J. Chem. Technol. Biotechnol. 92, 1891 (2017).
- T. Mahmood, M. Aslam, A. Naeem, T. Siddique, and S. U. Din, J. Chil. Chem. Soc. 63, 3855 (2018).
- A. Altinisik, Y. Seki, S. Ertas, E. Akar, E. Bozacı, and Y. Seki, Fibers Polym. 16, 370 (2015).
- Y. S. Ho and G. McKay, Process Biochem. 34, 451 (1999).
- Y. S. Ho and G. McKay, Chem. Eng. J. 70, 115 (1998).
- G. Schmuckler and S. Goldstein, in Ion Exch. Solvent Extr. A Ser. Adv. Vol. 7, edited by J. A. Marinsky and Y. Marcus, First (Dekker, New York, 1977), pp. 1–28.
- M. Caetano, C. Valderrama, A. Farran, and J. L. Cortina, J. Colloid Interface Sci. (2009).
- D. V. Morales, B. L. Rivas, and M. González, J. Chil. Chem. Soc. 61, 3295 (2016).
- B. Aşçı, E. Kövenç, Ö. Arar, and M. Arda, Glob. NEST J. 20, 368 (2018).
- Ö. Arar, Anadolu Univ. J. Sci. Technol. Appl. Sci. Eng. 17, 530 (2016).


