- Ruthenium(II) complex,
- Calf thymus DNA,
- Groove mode
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
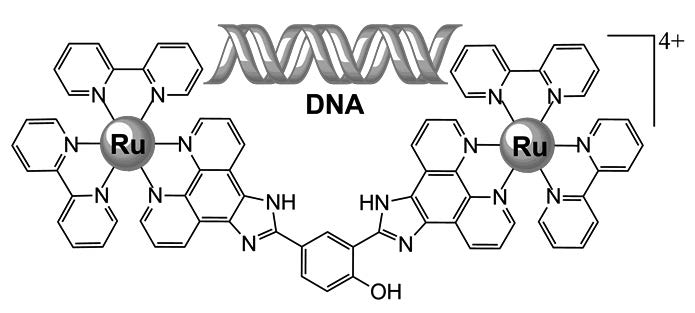
A dinuclear ruthenium complex [Ru2(bpy)4(bip-phenol)](ClO4)4 {bpy = 2,2′-bipyridine, bip-phenol = 2,4-bis(1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl) phenol} has been synthesized and characterized. The calf thymus (ct) DNA binding properties of the complex are investigated by means of DNA viscosity and optical spectroscopic techniques of UV-visible absorption and emission spectral titrations, steady-state emission quenching with ferrocyanide, ethidium bromide competitive binding, DNA thermal denaturation and reverse salt effect, together with molecular simulation technology. The results suggest that the complex is a promising DNA groove binder with a large DNA binding constant on 106 M−1 order of magnitude. The fluorescence of the complex manifests by 6.3-fold upon binding saturately to DNA. The complex is also demonstrated to be an efficient photocleaver of pBR 322 DNA.
References
- J.K. Barton, E.D. Olmon, P.A. Sontz, Coord. Chem. Rev. 255, 619-634, (2011).
- S.S. Bhat, V.K. Revankar, R.V. Pinjari, S. Naveen, C. Bogar, K. Bhat, V.A. Kawade, New J. Chem. 41, 5513-5520, (2017).
- H.K. Liu, P.J. Sadler, Acc. Chem. Res. 44, 349-359, (2011).
- M.R. Gill, J.A. Thomas, Chem. Soc. Rev. 41, 3179-3192, (2012).
- B.J. Pages, D.L. Ang, E.P. Wright, J.R. Aldrich-Wright, Dalton Trans. 44, 3505-3526, (2015).
- J.L. Morgan, C.B. Spillane, J.A. Smith, D.P. Buck, J.G. Collins, F.R. Keene, Dalton Trans. 10, 4333-4342, (2007).
- S.V. Sagar Babu, K.S.V. Krishna Rao, Y. Ill Lee, J. Chil. Chem. Soc. 62, 3447-3453, (2017).
- K. Margandan, S.J.M. Jebastin, J. Chil. Chem. Soc. 62, 3691-3699, (2017).
- J. Aldrich-Wright, C. Brodie, E.C. Glazer, N.W. Luedtke, L. Elson- Schwab, Y. Tor, Chem. Commun. 1018-1019, (2004).
- S.A. Ezadyar, A.S. Kumbhar, A.A. Kumbhar, A. Khan, Polyhedron 36, 45-55, (2012).
- C.C. Ju, A.G. Zhang, C.L. Yuan, X.L. Zhao, K.Z. Wang, J. Inorg. Biochem. 105, 435-443, (2011).
- C. Rajput, R. Rutkaite, L. Swanson, I. Haq, J.A. Thomas, Chem. Eur. J. 12, 4611-4619, (2006).
- H. Chao, Y.X. Yuan, F. Zhou, L.N. Ji, Trans. Met. Chem. 31, 465-469, (2006).
- P. Liu, B.Y. Wu, J. Liu, Y.C. Dai, Y.J. Wang, K.Z. Wang, Inorg. Chem. 55, 1412-1422, (2016).
- F.R. Liu, K.Z. Wang, G.Y. Bai, Y.A. Zhang, L.H. Gao, Inorg. Chem. 43, 1799-1806, (2004).
- J. Andersson, M. Li, P. Lincoln, Chem. Eur. J. 16, 11037-11046, (2010).
- A.G. Clark, M.N. Naufer, F. Westerlund, P. Lincoln, I. Rouzina, T. Paramanathan, M.C. Williams, Biochemistry 57, 614-619, (2018).
- A.A. Almaqwashi, T. Paramanathan, I. Rouzina, M.C. Williams, Nucleic Acids Res. 44, 3971-3988, (2016).
- A.A. Almaqwashi, T. Paramanathan, P. Lincoln, I. Rouzina, F. Westerlund, M.C. Williams, Nucleic Acids Res. 42, 11634-11641, (2014).
- J. Andersson, P. Lincoln, J. Phys. Chem. B 115, 14768-14775, (2011).
- V. Gonzalez, T. Wilson, I. Kurihara, A. Imai, J.A. Thomas, J. Otsuki, Chem. Commun. 1868-1870, (2008).
- X.L. Zhao, Z.S. Li, Z.B. Zheng, A.G. Zhang, K.Z. Wang, Dalton Trans. 42, 5764-5777, (2013).
- A. Ghosh, P. Das, M.R. Gill, P. Kar, M.G. Walker, J.A. Thomas, A. Das, Chem. Eur. J. 17, 2089-2098, (2011).
- L. Li, H.M. Liu, X.K. Liu, S.Y. Liao, Y.T. Lan, Q. Wu, X.C. Wang, Q. Wang, S.Y. Zhang, W.J. Mei, RSC Adv. 7, 23727-23734, (2017).
- Q. Wu, K.D. Zheng, S.Y. Liao, Y. Ding, Y.Q. Li, W.J. Mei, Organometallics 35, 317-326, (2016).
- Y.H. Chen, Q. Wu, X.C. Wang, Q. Xie, Y.Y. Tang, Y.T. Lan, S.Y. Zhang, W.J. Mei, Materials 9, 386, (2016).
- Q. Wu, T.F. Chen, Z. Zhang, S.Y. Liao, X.H. Wu, J. Wu, W.J. Mei, Y.H. Chen, W.L. Wu, L.L. Zeng, Dalton Trans. 43, 9216-9225, (2014).
- Z.B. Zheng, Q.Y. Huang, Y.F. Han, J. Zuo, Y.N. Ma, Sens. Actuat. B 253, 203-212, (2017).
- V. Muthuraj, M. Umadevi, J. Mol. Struct. 1157, 201-209, (2018).
- W.X. Hong, H.W. Huang, T.W. Huang, X. Xu, Q.G. Han, G.F. Wang, H. Xu, S. Duan, Y.H. Duan, X. Long, Y. Liu, Z.L. Hu, J. Inorg. Biochem. 180, 54-60, (2018).
- M. Mohanraj, G. Ayyannan, G. Raja, C. Jayabalakrishnan, Mat. Sci. Eng. C-Mater. 69, 1297-1306, (2016).
- C.W. Jiang, J. Inorg. Biochem. 98, 497-501, (2004).
- V. Muthuraj, M. Umadevi, J. Mol. Struct. 1157, 201-209, (2018).
- D. Suh, J.B. Chaires, Bioorg. Med. Chem. 3, 723-728, (1995).
- A.G. Zhang, Y.Z. Zhang, Z.M. Duan, K.Z. Wang, H.B. Wei, Z.Q. Bian, C.H. Huang, Inorg. Chem. 50, 6425-6436, (2011).
- J.Z. Wu, L. Yuan, J. Inorg. Biochem. 98, 41-45, (2004).
- F.M. O’Reilly, J.M. Kelly, New J. Chem. 22, 215-217, (1998).
- S. Mardanya, S. Karmakar, D. Mondal, S. Baitalik, Inorg. chem. 55, 3475- 3489, (2016).
- S.J. Burya, D.A. Lutterman, C. Turro, Chem. Commun. 47, 1848-1850, (2011).
- M.M. Milutinović, A. Rilak, I. Bratsos, O. Klisurić, M. Vraneš, N. Gligorijević, S. Radulović, Ž.D. Bugarčić, J. Inorg. Biochem. 169, 1-12, (2017).
- R.B. Nair, E.S. Teng, S.L. Kirkland, C.J. Murphy, Inorg. Chem. 37, 139- 141, (1998).
- L. Xu, Y. Chen, H. Wei, W.S. Wu, Z.Z. Li, Y.W. Lin, H. Chao, L.N. Ji, Chem. Res. Chin. Univ. 30, 461-467, (2014).
- A. Ambroise, B.G. Maiya, Inorg. Chem. 39, 4264-4272, (2000).
- F. Gao, H. Chao, F. Zhou, Y.X. Yuan, B. Peng, L.N. Ji, J. Inorg. Biochem. 100, 1487-1494, (2006).
- J.M. Kelly, A.B. Tossi, D.J. McConnell, C. OhUigin, Nucleic Acids Res. 13, 6017-6034, (1985).
- Y.J. Liu, W.J. Mei, J.Z. Lu, H.J. Zhao, L.X. He, F.H. Wu, J. Coord. Chem. 61, 3213-3224, (2008).
- X.L. Hong, H. Chao, L.J. Lin, K.C. Zheng, H. Li, X.L. Wang, F.C. Yun, L.N. Ji, Helv. Chim. Acta. 87, 1180-1193, (2004).
- S. Satyanarayana, J.C. Dabrowiak, J.B. Chaires, Biochemistry 32, 2573- 2584, (1993).
- F. Leng, J.B. Chairs, M.J. Waring, Nucleic Acids Res. 31, 6191-6197, (2003).
- N.M. Gabra, B. Mustafa, Y.P. Kumar, C.S. Devi, A. Srishailam, P.V. Reddy, K.L. Reddy, S. Satyanarayana, J. Fluoresc. 24, 169-181, (2014).
- Mudasir, K. Wijaya, E.T. Wahyuni, N. Yoshioka, H. Inoue, Biophys. Chem. 121, 44-50, (2006).
- F. Pierard, A.D. Guerzo, A.K. Mesmaeker, M. Demeunynck, J. Lhomme, Phys. Chem. Chem. Phys. 3, 2911-2920, (2001).
- S. Satyanarayana, J.C. Dabrowiak, J.B. Chaires, Biochemistry 31, 9319- 9324, (1992).
- X.W. Liu, Y.M. Shen, Z.X. Li, X. Zhong, Y.D. Chen, S.B. Zhang, Spectrochim. Acta A 149, 150-156, (2015).
- C.W. Jiang, Eur. J. Inorg. Chem. 2004, 2277-2282, (2004).
- Y.N. Liu, T.F. Chen, Y.S. Wong, W.J. Mei, X.M. Huang, F. Yang, J. Liu, W.J. Zheng, Chem. Biol. Interact. 183, 349-356, (2010).
- Y.P. Kumar, C.S. Devi, A. Srishailam, N. Deepika, V.R. Kumar, P.V. Reddy, J. Fluoresc. 26, 2119-2132, (2016).


