- Gamma-irradiation,
- Microspheres,
- PLGA,
- Rasagiline mesylate,
- Parkinson’s disease
Copyright (c) 2017 Marcos Fernández, Emilia Barcia, Sofía Negro

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
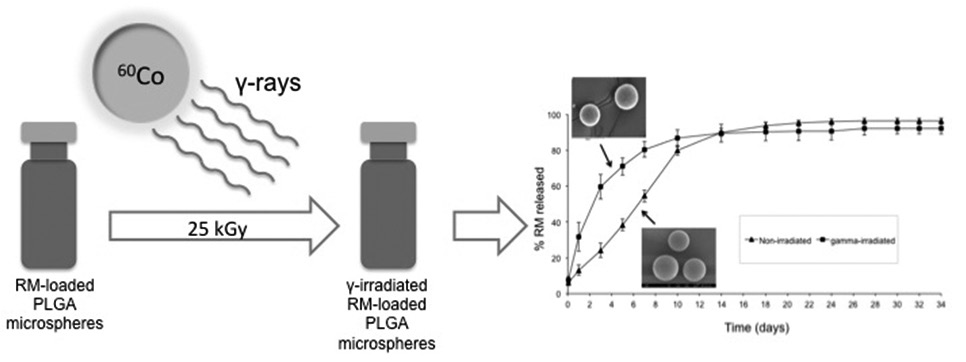
In the present study, the influence of gamma-irradiation was evaluated on the physicochemical characteristics and in vitro release of rasagiline mesylate (RM), a selective MAO-B inhibitor used in Parkinson’s disease, from poly(D,L-lactide-co-glycolide) (PLGA) microspheres. Microspheres were prepared using PLGA 50:50 by the solvent evaporation technique (O/W emulsion). Microspheres were sterilized by gamma-irradiation and their influence was assessed by scanning electron microscopy (SEM), laser light diffraction, differential scanning calorimetry (DSC), X-ray diffraction (XRD), gel permeation chromatography (GPC), encapsulation efficiency (EE) and in vitro drug release. Gamma-irradiation of RM-loaded microspheres did not affect EE, DSC and XRD patterns. After gamma-irradiation, changes on the surface were observed by SEM, but no significant difference in mean particle size was observed. GPC measurements showed a decrease in molecular weight of the polymer after five days of in vitro release. The similarity factor value between irradiated and non-irradiates microspheres was <50, indicating the non-similarity of the release profiles. The sterilization technique had an effect on the integrity of polymeric system, significantly affecting in vitro release of RM from PLGA microspheres. Therefore, from our results we conclude that gamma-irradiation is not a suitable sterilization procedure for this formulation.
References
- D. Tarsy, JAMA 307, 2305 (2012).
- B. Connolly, A. Lang, JAMA 311, 1670 (2013).
- M. Youdim, A. Gross, J. Finberg, Br. J. Pharmacol. 132, 500 (2001).
- C.W. Olanow, O. Rascol, R. Hauser, P.D. Feigin, J. Jankovic, A. Lang, W. Langston, E. Melamed, W. Poewe, F. Stocchi, E. Tolosa E., ADAGIO Study Investigators, N. Engl. J. Med. 361, 1268 (2009).
- J. Chen, D. Swope, K. Dashtipour, Clin. Ther. 29, 1825, (2007).
- J. Anderson, M. Shive, Adv. Drug Deliver. Rev. 28, 5 (1997).
- J. Choi, K. Seo, J. Yoo, J. Pharm. Inv. 42, 155 (2012).
- J.P. Benoit, N. Faisant, M.C. Venier-Julienne, P. Menei, J. Control. Release 65, 285 (2000).
- European Guideline 3AQ4a. 1992. “The Use of Ionising Radiation in the Manufacture of Medicinal Products (6/1992), Official Publications of the Communities”: áhttp://www.ema.europa.eu/docs/en_GB/document_ library/Scientific_guideline/2009/09/WC500002918.pdfñ, cited 20 August, 2014.
- C. Martínez-Sancho, R. Herrero-Vanrell, S. Negro, J. Control. Release 99, 41 (2004).
- A. Hausberger, R. Kenley, P. DeLuca, Pharm. Res. 12, 851 (1995).
- L. Montanari, M. Constantini, E. Signoretti, L. Valvo, M. Santucci, M. Bartolomei, P. Fattibene, S. Onori, A. Faucitano, B. Conti, I. Genta, J. Control. Release 56, 219 (1998).
- B. Bittner, K.Mäder, C. Kroll, H. Borchert, T. Kissel, J. Control. Release 59, 23 (1999).
- R. Dorati, C. Colonna, M. Serra, I. Genta, T. Modena, F. Pavanetto, P. Perugini, B. Conti, AAPS PharmSciTech. 9, 718 (2008).
- M. Gupta, V. Deshmukh, Polymer 24, 827 (1983).
- C. Volland, M. Wolff, T. Kissel, J. Control. Release 31, 293 (1994).
- M. Fernández, S. Negro, K. Slowing, A. Fernández-Carballido, E. Barcia, Int. J. Pharm. 419, 271 (2011).
- M. Fernández, E. Barcia, S. Negro, J. Pharm. Biomed. Anal. 49, 1185 (2009).
- X. Xiao, L. Chu, W. Chen, J. Zhu, Polymer 46, 3199 (2005).
- V. Shah, Y. Tsong, P. Sathe, L. Jen-Pei, Pharm. Res. 15, 889 (1998).
- M. Claybourn, H. Gray, D. Murphy, I. Purnell, C. Rowlands, J. Control. Release 91, 431 (2003).
- S. Caliş, S. Bozdag, H. Kaş, M. Tunçay, A. Hincal, Farmaco 57, 55 (2002).
- L. Woo, C. Sandford, Radiat. Phys. Chem. 63, 845 (2002).
- A. Shenderova, T. Burke, S. Schwendeman, Pharm. Res. 16, 241 (1999).
- M. Dunne, I. Corrigan, Z. Ramtoola, Biomaterials 21, 1659 (2000).
- P. Sansdrap, A. Moës, J. Control. Release 43, 47 (1997).
- M. Sintzel, K. Schwach-Abdellaoui, K. Mäder, R. Stösser, J. Heller, C. Tabatabay, R. Gurny, Int. J. Pharm. 175, 165 (1998).
- J. Nijsen, A. van Het Schip, M. van Steenbergen, S. Zielhuis, L. Kroon- Batenburg, M. van de Weert, P. van Rijk, W. Hennink, Biomaterials 23, 1831 (2002).
- L. Montanari, F. Cilurzo, F. Valvo, A. Faucitano, A. Buttafava, A. Groppo, I. Genta, B. Conti, J. Control. Release 75, 317 (2001).
- H. Okada, Adv. Drug Deliver. Rev. 28, 43 (1997).
- C. Carrascosa, L. Espejo, S. Torrado, J. Torrado, J. Biomater. Appl. 18, 95 (2003).
- L. Montarani, F. Cilurzo, F. Selmin, B. Conti, I. Genta, G. Poletti, F. Orsini, L. Valvo, J. Control. Release 90, 281 (2003).
- S. Yoshioka, Y. Aso, S. Kojima, J. Control. Release 37, 263 (1995).
- G. Spenlehauer, M. Vert, J.P. Benoit, F. Chabot, M. Veillard, J. Control. Release 7, 217 (1988).
- J. Ruiz, J. Busnel, J.P. Benoit, Pharm. Res. 7, 928 (1990).
- S. Yoshioka, Y. Aso, T. Otsuka, S. Kojima, Radiat. Phys. Chem. 46, 281 (1995).


