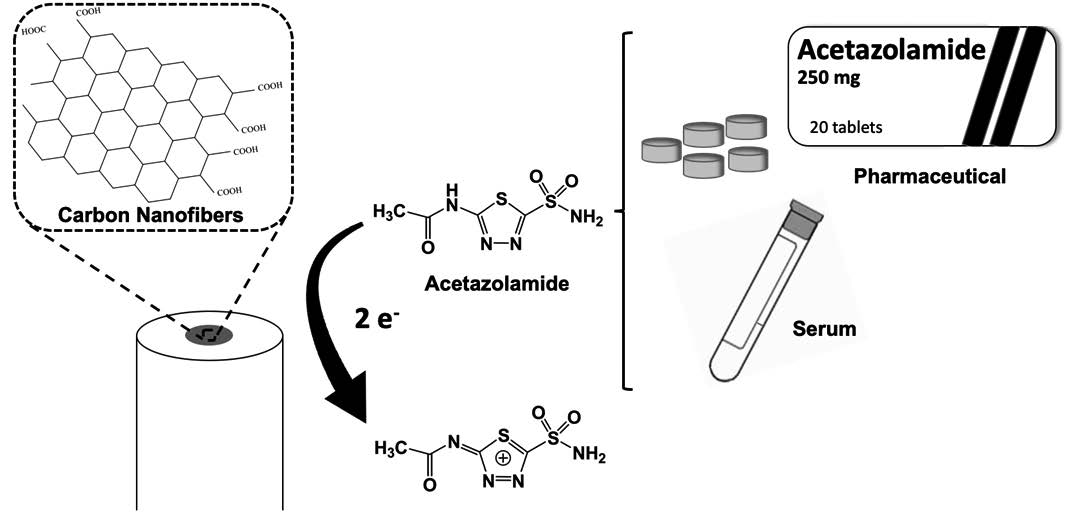
DEVELOPMENT AND CHARACTERIZATION OF A SENSOR BASED ON CARBON NANOFIBERS: APPLICATION TO ACETAZOLAMIDE DETERMINATION IN PHARMACEUTICALS AND BIOLOGICAL FLUIDS
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Acetazolamide (ACZ) is a carbonic anhydrase inhibitor that exhibits diuretic activity. In medicine, it is principally used in open-angle glaucoma, for prevention or amelioration of symptoms associated with acute high-altitude sickness and as an adjunct to other anticonvulsants in centrencephalic epilepsies. Furthermore, ACZ is sometimes used by athletes to mask the presence of doping substances, so its use has been banned in competitions.
The aim of this work was the development of a sensor to determine ACZ in pharmaceuticals, as quality control way, and serum, for clinical and doping analyzes. The sensor has been developed modifying a glassy carbon electrode with carbon nanofibers.
The electrochemical characterization of the sensor was performed by cyclic voltammetry using the redox mediator potassium ferrocyanide. ACZ electrochemical behavior was studied as well.
Measurements were performed in a conventional three electrodes cell using differential pulse voltammetry. The influence of some experimental variables involved in the preparation and performance of the sensor –amount of modifier, supporting electrolyte, pH, scan rate and pulse amplitude– were optimized.
This methodology provided a linear calibration plot for ACZ in the 1 – 17 μM concentration range. Furthermore, the proposed sensor exhibited suitable analytical properties, with detection and quantification limits of 0.06 μM and 0.2 μM respectively; analytical sensitivity of 1.142 μA/μM, repeatability of 0.72 %, and it was applied to determine ACZ in pharmaceuticals (tablets) and spiked human serum, being an excellent tool to perform quality control and antidoping control.
References
- American Society of Health-System, AHFS Drug Information, AHSP, Maryland:, 2012.
- World Anti-doping Agency, “The 2014 Prohibited List,” Canada, 2014.
- R. Shingles, J. Marquey, Anal. Biochem, 252, 19, (2001).
- [4] Z. Gómez de Baluguera, et al.,Journal Pharm. Biomed. Anal., 12, 883, (1994).
- [5] S. M. Wallace, et al., J. Pharm. Sci, 66, 527, (1977).
- [6] The United States Pharmacopeia Convention, Farmacopea de los Estados Unidos de América (USP), 35, 2012.
- [7] E. Garrido, J. Garrido, F. Borges, C. Delerue-Matos, Journal of Pharma¬ceutical and Biomedical Analysis, 32, 975, (2003).
- [8] I. Süslü, S. Altinoz, Journal of Pharmaceutical and Biomedical Analysis, 39, 535, (2005).
- [9] T. Almeida-Silva, H. Zanin, F. Campanha Vicentini, E. Corat, O. Fatibel¬lo-Filho, Sensors and Actuators B, 218, 51, (2015).
- [10] T. Lu, Y. Tsai, Sensors and Actuators B, 153, 439, (2011).
- [11] R. Fernández Torres, M. Callejon Mochon, J. Jimenez Sanhez, M. Bello Lopez, A. Guiraum Perez, Journal of Pharmaceuticals and Biomedical Analysis, 30, 1215, (2002).
- [12] R. Goyal, A. Rana, H. Chasta, “Bioelectrochemistry, 83, 46, (2012).
- [13] M. Sadikovic, B. Nigovic, S. Juric, A. Mornar, Journal of Electroanalyti¬cal Chemistry, 733, 60, (2014).
- [14] A. Afkhami, F. Soltani-Felehgari, T. Madrakian, Electrochimica acta, 103, 125, (2013).
- [15] L. Svorc, K. Cinkova, J. Sochr, M. Vojs, P. Michniak, M. Marton, Journal of Electroanalytical Chemistry, 728, 86, (2014).
- [16] W. Silva Machini, M. Texeira, Colloquium Exactarum, 6, 45, (2014).
- [17] Y. Dzenis, Science, 304, 25, 1917, (2004).
- [18] V. Vamvakaki, K. Tsagaraki, N. Chaniotakis, Anal. Chem., 78, 5538, (2006).
- [19] N. M. Rodríguez, Journal of Materials Research, 8, 3233, (1993).
- [20] A. Eitan, K. Jiang, D. Dukes, et al., Chemistry of Materials, 15, 3198, (2003).
- [21] R. Mundaca-Uribe, F. Bustos-Ramirez, C. Zaror-Zaror, et al., Sensors and Actuators B, 195, 58, (2014).


