- Applications,
- enzymatic immobilization,
- levansucrase,
- supports
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
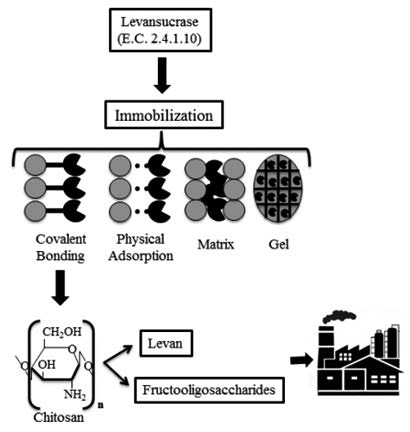
Immobilization is an excellent tool for enzymatic stabilization, improving the biocatalytic processes, allowing the reuse of the enzyme and promoting an easier separation of the molecule of interest. Currently, new enzymatic bonding processes are arising on solid supports, based on classical immobilization methods. Amongst the supports used, chitosan is a polysaccharide that offers a unique set of characteristics, as biocompatibility, biodegradability, non-toxicity and antibacterial properties. Thus, many enzymes has being immobilized on this support, including levansucrase, that is able to synthesize levan and fructooligosaccharides, two important biomolecules which have beneficial health properties. These review present different methods of immobilization (physical adsorption, entrapment, crosslinking and covalent bonding) for fructosyltransferases, as well as different immobilization matrices that can be applied in biotechnological processes. However, studies are still needed in order to adopt efficient immobilization techniques, in which the biocatalyst remains more stable, in order to become the process attractive to the industrial sector.
References
- E. P. Cipolatti, A.Valério, R. O. Henriques, D. E. Moritz, J. L. Ninow, D. M. G. Freire, D. Oliveira. RSC Adv. 6, 104675–104692, (2016).
- L. Canilha, W. Carvalho, J. B. A. Silva. Biotecnologia, ciência e Desenvolvimento ano IX, 36, 48–57, (2006).
- N. R. Mohamad, N. H. C. Marzuki, N. A. Buang, F. Huyop, R. A. Wahab, Biotechnol. Biotechnol. Equipm. 29, 205–220, (2015).
- S. Gao, Y. Wang, X. Diao, G. Luo, Y. Dai. Bioresour. Technol. 101, 3830– 3837, (2010).
- P. Zucca, R. Fernandez-Lafuente, E. Sanjust, Molecul. 21, 1–25, (2016).
- W. Li, S. Yu, T. Zhang, B. Jiang, W. Mu, Appl. Microbiol. Biotechnol. 99, 6959–6969, (2015).
- Y. Ben Ammar, T. Matsubara, K. Ito, M. Iizuka, N. Minamiura, Enzyme Microbial. Technol. 30, 875–882, (2002).
- K. Le Gorrec, C. Connes, A. Guibert, J.L. Uribelarrea, D. Combes. Enzyme Microbial Technol. 31, 44–52, (2002).
- B. L. Cantarel, P. M. Coutinho, C. Rancurel, T. Bernard, V. Lombard, B. Henrissat, Nucleic acids research. 37, 233–238, (2009).
- J. W. Yun. Enzyme Microbial Technol. 19, 107–117, (1996).
- F. Guio, L. D. Rugeles, S. E. Rojas, M. P. Palomino, M. C. Camargo, O. F. Sánchez. Appl. Biochem. Biotechnol. 167, 142–63, (2012).
- D. Linde, B. Rodríguez-Colinas, M. Estévez, A. Poveda, F. J. Plou, M. Fernández Lobato. Bioresour. Technol. 109, 123–130, (2012).
- P. Santos-Moriano, L. Fernandez-Arrojo, A. Poveda, J. Jimenez-Barbero, A. O. Ballesteros, F. J. Plou. J. Molecul. Catalysis B: Enzym. 119, 18–25, (2015).
- R. Srikanth, C. H. S. S. S. Reddy, G. Siddartha, M. J. Ramaiah, K. B. Uppuluri, Carbohydr. Polymers, 120, 102–114, (2015).
- F. T. Tian, L. Inthanavong, S. Karboune. 75, 1929–1938, (2011).
- G. T. Bersaneti, N. C. Pan, C. Baldo, M. A. P. C. Celligoi, Appl. Biochem. Biotechnol. 184, 838-851 (2017).
- L. Inthanavong, F. Tian, M. Khodadadi, S. Karboune, Biotechnol. Progr. 29, 1405–1415, (2013).
- Wuerges, J. Caputi, L. Cianci, M. Boivin, S. Meijers, R. Benini, S. J. Struct. Biol. 191, 290-298, (2015).
- J. Seibel, R. Moraru, S. Götze, K. Buchholz, S. Na’amnieh, A. Pawlowski, H. J. Hecht, Carbohydr. Resear. 341, 2335–2349, (2006).
- G. Meng, K. Fütterer. Nat. Struct. Biol. 10, 935–41, (2003).
- K. S. Belghith, I. Dahech, H. Belghith, H. Mejdoub, Int. J. Biol. Macromol. 50, 451–8, (2012).
- P. B. Silva, D. Borsato, M. A. P. C. Celligoi. Afr. J. Biotechnol. 13, 2734– 2740, (2014).
- B. C. M. Gonçalves, J. Mantovan, M. L. L. Ribeiro, D. Borsato, M. A. P. C. Celligoi. J. Appl. Biol. Biotechnol. 1, 9–12, (2013).
- P. Santos-Moriano, L. Monsalve-Ledesma, M. Ortega-Munoz, L. Fernandez-Arrojo, A. O. Ballesteros, F. Santoyo-Gonzalezb, F. J. Plou. RSC Adv. 6, 64175–64181, (2016).
- L. Fernandez-Arrojo, B. Rodriguez-Colinas, P. Gutierrez-Alonso, M. Fernandez-Lobato, M. Alcalde, A. O. Ballesteros, F. J. Plou. Process Biochem. 48, 677–682, (2013).
- M. A. Esawy, D. A. R. Mahmoud, A. F. A. Fattah. Braz. J. Chem. Eng 25, 237–246, (2008).
- K. H. Jang, K. B. Song, J. S. Kim, C. H. Kim, B. H. Chung, S. K. Rhee, Bioprocess Eng. 23, 89–93, (2000).
- K. R. Jegannathan, S. Abang, D. Poncelet, E. S. Chan, P. Ravindra. Crit. Rev. Biotechnol. 28, 253–264, (2008).
- A. A. Mendes, P. C. Oliveira, H. F. Castro, R. L. C. Giordano. Quim. Nova. 34, 831–840, (2011).
- S. Karav, J. L. Cohen, D. Barile, J. M. L. N. de Moura Bell, Biotechnol. Progress. 33, 104–112, (2017).
- K. Okuda, I. Urabe, Y. Yamada, H. Okada. J. Ferment. Bioeng. 71, 100– 105, (1991).
- S. Datta, L. R. Christena, Y. R. S. Rajaram. 3 Biotech, 3, 1–9, 2013.
- R. Dalla-Vecchia, M. D. G. Nascimento, V. Soldi. Quim. Nova, 27, 623– 630, (2004).
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan, R. Fernandez-Lafuente. Enzyme Microb. Technol. 40, 1451–1463, (2007).
- M. L. Foresti, M. L. Ferreira, Enzyme Microb. Technol. 40, 769–777, (2007).
- C. Garcia-Galan, Á. Berenguer-Murcia, R. Fernandez-Lafuente, R. C. Rodrigues, Adv. Synth. Catal. 353, 2885–2904, (2011).
- J.G.C. Pradella, Reatores com células imobilizadas. Biotecnologia Industrial. W. Schmidell, U.A. Lima, E. Aquarone, W. Borzani, São Paulo: Ed. Edgard Blücher, 2001; cap.16; pp.355- 372.
- A. Hill, S. Karboune, C. Mateo, Process Biochem. 61, 63–72, (2017).
- S. Sangmanee, S. Nakapong, R. Pichyangkura, K. Kuttiyawong. J. Scienc. Technol. 38, 295–303, (2016).
- A. Hill, S. Karboune, C. Mateo. J. Chem. Technol. Biotechnol. 91, 2440– 2448, (2015).
- A. S. G. Lorenzoni, L. F. Aydos, M. P. Klein, M. A. Z. Ayub, R. C. Rodrigues, P. F. Hertz. J. Mol. Catal. B: Enzym. 111, 51–55, (2015).
- I. Dahech, R. Bredai, K. Srih. Biochem. 8, 2013–2014, (2014).
- F. Tian, S. Karboune, A. Hill. Innov. Food Sci. Emerg. Technol. 22, 230– 238, (2014).
- K.H. Jang, K. B. Song, B. S. C. H. Park, Kim, B. H. Chung, R. W. Choue, S.K. Rhee. Process Biochem. 37, 339–343, (2001).
- V. Zargar, M. Asghari, A. Dashti. Chem. Bio. Eng. Reviews. 2, 204–226, (2015).
- V. K. Thakur, M. K. Thakur. ACS Sustain. Chem. Eng. 2, 2637–2652, (2014).
- M. I. Wahba. Int. J. Biol. Macromol. 105, 894–904, (2017).
- R. Muzzarelli, G. Barontini, R. Rocchetti. Biotechnol. Bioeng. 18, 1445– 54, (1976).
- T. Kasumi, M. Tsuji, K. Hayashi, N. Tsumura, Agric. Biol. Chem. 41, 1865–1872, (1977).
- M. A. Ganaie, A. Lateef, U. S. Gupta. Appl. Biochem. Biotechnol. 172, 2143–59, (2014).
- S. Hama, S. Tamalampudi, A.Yoshida, N. Tamadani, N. Kuratani, H. Noda, H. Fukuda, A. Kondo. Biochem. Eng. J. 55, 66–71, (2011).
- E. Ricca, V. Calabrò, S. Curcio, A. Basso, L. Gardossi, G. Iorio, Int. J. Mol. Sci. 11, 1180–9, (2010).


