- tetrasilacyclobutadiene,
- solvent effect,
- frontier orbitals,
- natural bond analysis (NBO)
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
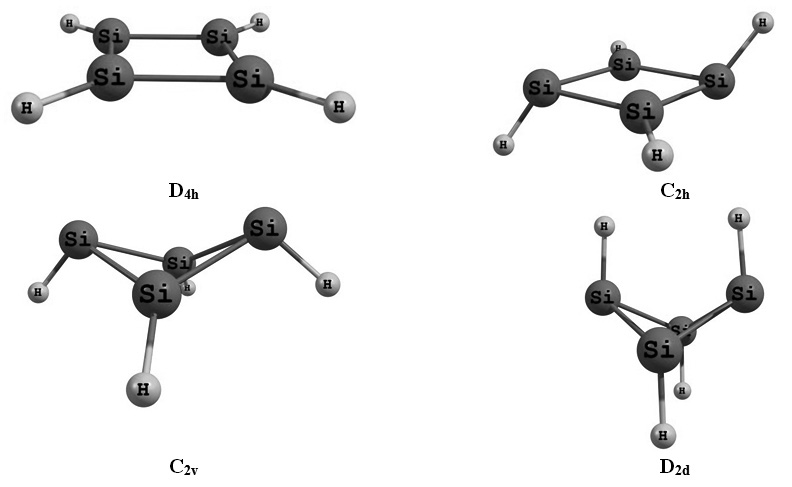
In this investigation, we was explored solvent influence on the stability and properties of different isomers of Si4H4 molecule in both gas and solution phases. Our calculation was performed at the M062X/Def2-TZVPP level of theory. For solution phase calculations, self-consistent reaction field (SCRF) approach was used by the polarizable continuum model (PCM). The solvation model applied the radii and non-electrostatic terms of the solvent model density (SMD). The eight selected solvents were Chloroform, o-NitroToluene, CycloHexanone, TetraHydroFuran, n,n-DiMethylFormamide, DiMethylDiSulfide, PropanoNitrile and DiChloroEthane. The stability of the isomers were investigated in both phases and the solvation energy values of them were calculated. Solvent effect on the frontier orbital energy and HOMO-LUMO gap was clarified. The most instance vibration of the most stable isomer was determined and solvent influence on the wavenumber of this vibration was explored. Lastly, natural bond analysis (NBO) was used for the illustration of the Si-Si chemical bonds, strongest interaction and natural atomic charges of the most stable isomer.
References
- S. Inoue, J. D. Epping, E. Irran, M.Driess, J. Am. Chem. Soc. , 133, 8514 (2011).
- S.-H. Zhang, H.-W. Xi, K. H. Lim, C.-W. So, V. Y. Lee, Y. Ito, Angew. Chem. Int. Edit., 52, 12364 (2013).
- V. Y. Lee, Y. Ito, H. Yasuda, K. Takanashi, A. Sekiguchi, J. Am. Chem. Soc. , 133, 5103 (2011).
- e. a. H. X. Yeong, Chem. Eur. J., 18, 2685 (2012).
- R. F. Gunion, H. Koppel, G. W. Leach, W. C. Lineberger, J. Chem. Phys., 103, 1250 (1995).
- F. Nazari, Z. T. Doroodi, Int. J. Quantum Chem., 110, 1514 (2010).
- P. Selvarengan, P.Kolandaivel, J Mol Struct: THEOCHEM, 617, 99 (2002).
- S. B. Allin, T. M.Leslie, R. S. Lumpkin, Chem Mater 8, 428 (1996).
- A. J. A. Aquino, D. Tunega, G. Haberhauer, M. H. Gerzabek, H. Lischka, J Phys Chem A, 106, 1862 (2002).
- M. Springborg, Solvation effects, in: Chemical Modelling: Applications and Theory Volume 5, The Royal Society of Chemistry, 2008, pp. 67-118.
- J. Tomasi, B. Mennucci, R. Cammi, Chem. Rev., 105, 2999 (2005).
- M. Rezazadeh, R. Ghiasi, S. Jamehbozorgi, J. Struc. Chem, 59, 245 (2018).
- R. Ghiasi, F.Zafarniya, S. Ketabi, Russian Journal of Inorganic Chemistry, 62, 1371 (2017).
- H. Alavi, R. Ghiasi, J. Struc. Chem, 58, 30 (2017).
- F. Zafarniya, R.Ghiasi, S. Jameh-Bozorghi, Physics and Chemistry of liquids, 55, 444 (2017).
- F. Zafarnia, R. Ghiasi, S. Jamehbozorgi, J. Struc. Chem, 58, 1324 (2017).
- N. Sadeghi, R. Ghiasi, R. Fazaeli, S. Jamehbozorgi, Journal of Applied spectroscopy 83, 909 (2016).
- R. Ghiasi, A. Peikari, Physical and Chemistry of Liquids, 55, 421 (2017).
- R. Ghiasi, A. Peikari, Russian Journal of Physical Chemistry A, 90, 2211 (2016).
- R. Ghiasi, A. Peikari, Journal of Applied Spectroscopy 84, 148 (2017).
- R. Ghiasi, H. Pasdar, S. Fereidoni, Russian Journal of Inorganic Chemistry, 61, 327 (2016).
- R. Ghiasi, M. Nemati, A. H.Hakimioun, J. Chil. Chem. Soc, 61, 2921 (2016).
- A. Peikari, R. Ghiasi, H. Pasdar, Russian Journal of Physical Chemistry A, 89, 250 (2015).
- R. Ghiasi, E. Amini, Journal of Structural Chemistry, 56, 1483 (2015).
- M. Z. Fashami, R. Ghiasi, Journal of Structural Chemistry, 56, 1474 (2015).
- M. Rezazadeh, R. Ghiasi, S. Jamehbozorgi, Journal of Applied Spectroscopy, 85, 926 (2018).
- F. Rezaeyani, R. Ghiasi, M. Yousefi, Russian Journal of Physical Chemistry A, 92, 1748 (2018).
- M. Rahimi, R. Ghiasi, Journal Molecular Liquid, 265, 164 (2018).
- R. Ghiasi, Journal Molecular Liquid, 264, 616 (2018).
- D. Li, Y. Wanga, K. Han, Coordination Chemistry Reviews, 256, 1137 (2012).
- G. Song, Y. Su, R. A. Periana, R. H. Crabtree, K. Han, H. Zhang, X. Li, Angewandte Chemie International Edition, 49, 912 (2010).
- H. Wang, Y. Wang, K.-L. Han, X.-J. Peng, J. Org. Chem., 70, 4910 (2005).
- D. Li, X. Huang, K. Han, C.-G. Zhan, J. Am. Chem. Soc., 133, 7416 (2011).
- R. Ghiasi, M. Z. Fashami, Journal of Theoretical and Computational Chemistry, 13, 1450041-1 (2014).
- N. Shajari, R. Ghiasi, Journal of Structural Chemistry, 59, 541 (2018).
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalman, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox. (2009). Gaussian 09 (Version Revision A.02). Wallingford CT: Gaussian, Inc.
- F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys., 7, 3297 (2005).
- Y. Zhao, D. G. Truhla, J. Phys. Chem. A, 110, 5121 (2006).
- A. E. Reed, L. A. Curtiss, F. Weinhold, Chem. Rev. , 88, 899 (1988).
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales, C. R. Landis, F. Weinhold. (2013). NBO 6.0. . Theoretical Chemistry Institute, University of Wisconsin, Madison, WI,.
- J. Tomasi, B. Mennucci, R. Cammi, Chem. Rev., 105, 2999 (2005).
- A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. B 113, 6378 (2009).
- P. W. Ayers, R. G. Parr, J. Am. Chem. Soc. , 422, 2010 (2000).
- R. G. Parr, P. K. Chattaraj, J. Am. Chem. Soc. , 113, 1854 (1991).
- R. G. Pearson, J. Chem. Educ. , 64, 561 (1987).
- R. G. Pearson, Acc. Chem. Res. , 26, 250 (1993).
- R. G. Pearson, J. Chem. Educ., 76, 267 (1999).


