EXPERIMENTAL AND THEORETICAL ANALYSIS OF N,N’-(ETHANE-1,2-DIYLBIS(4,1-PHENYLENE)) BIS(1-(THIOPHEN-2-YL)METHANIMINE) AND N,N’-(ETHANE-1,2-DIYLBIS(4,1-PHENYLENE))BIS(1-(4- METHYLTHIOPHEN-2-YL)METHANIMINE) SCHIFF BASE LIGANDS
- thiophene,
- schiff base,
- Gaussian 09w,
- DFT/B3LYP
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
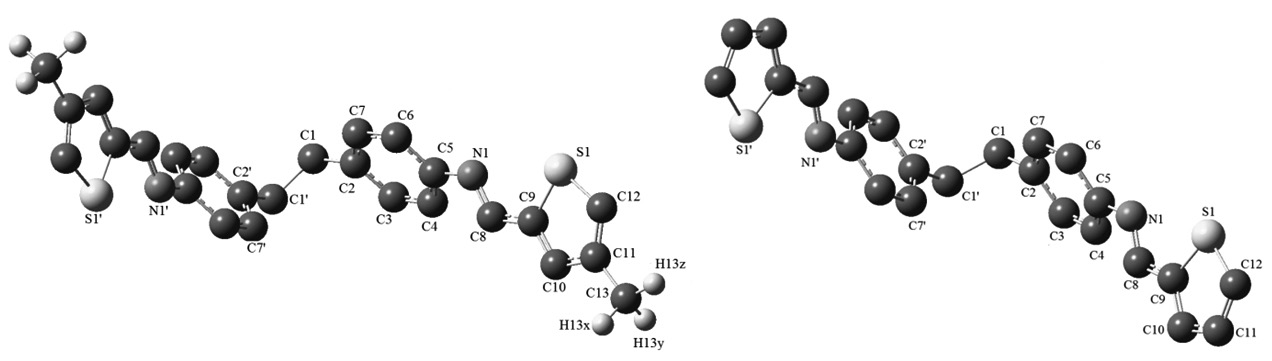
N,N’-(ethane-1,2-diylbis(4,1-phenylene))bis(1-(thiophen-2-yl)methanimine) and N,N’-(ethane-1,2-diylbis(4,1-phenylene))bis(1-(4-methylthiophen-2-yl) methanimine) ligands are formed by diamine and two aromatic aldehyde using Schiff base condensation method. Ligands are characterised by fourier transform infrared spectroscopy (FT-IR), 1H- and 13C- nuclear magnetic resonance spectroscopy (1H- and 13C- NMR) and mass spectroscopy (LC ESI/MS) methods. Furthermore, geometric properties such as bond lenghts, bond angles, dihedral angles, electronic properties, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies are calculated by using Gaussian 09w program. Experimental and theoretical spectrum datas are compared.
References
- C. Chandramouli, M. R. Shivanand, T. B. Nayanbhai, B. Bheemachari, R.H. Udupi, J Chem Pharm Res, 4, 1151, (2012).
- R.P. Chinnasamy, R. Sundararajan, S. Govindaraj, J Adv Pharm Technol Res, 1, 342, (2010).
- R. Miri, N. Razzaghi-asl, M.K. Mohammadi, Journal of molecular modeling, 19, 727, (2013).
- P. Venkatesh, Asian J Pharm Health Sci, 1, 8, (2011).
- D. Wei, N. Li, G. Lu, K. Yao, 49, 225, (2006).
- A. Pui, T. Malutan, L. Tataru, C. Malutan, D. Humelnicu, G. Carja, Polyhedron, 30, 2127, (2011).
- D.M. Boghaei, S. Mohebi, Tetrahedron, 58, 5357, (2002).
- S. Mihai, M. Negoiu, A. Bondarev, Rev. Chım. (Bucuresti), 60, 778, (2009).
- A. Yaul, G. Pethe, R. Deshmukh, A. Aswar, J. Therm. Anal. Calorim., 113, 745, (2013).
- J.R. Zamian, E.R. Dockal, Tran. MetalChem., 21, 370, (1996).
- R.A. Smith, S. Natelson, 53, 3476, (1931).
- R.G. Ramsinghani, Z.A. Filmwala, WJPPS, 6, 1255, (2017).
- I. Alkorta, J.J. Perez, Int. J. Quantum Chem., 57, 123, (1996).
- C. James, C. Ravikumar, T. Sundius, V. Krishnakumar, R. Kesavamoorthy, V.S. Jayakumar, I. Hubert Joe, Vib. Spectrosc. , 47, 10, (2008).
- D.F.V. Lewis, H.B. Broughton, ScientificWorldJournal., 27, 1776, (2002).
- J.S. Murray, K. Sen (1996) Molecular Electrostatic Potentials, Concepts and Applications. 1 edn. Elsevier Science, Amsterdam-Netherlands.
- R.G. Pearson, Proc. Natl. Acad. Sci. U. S. A., 83, 8440, (1986).
- J. Šponer, P. Hobza, Int. J. Quantum. Chem., 57, 959, (1996).
- A. Vela, J.L. Gazquez, Am. Chem. Soc., 112, 1490, (1990).
- M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery, J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox (2009) Gaussian 09, Revision B.01. Wallingford CT.
- L. Casella, J.A. Ibers, Inorg. Chem., 20, 2438, (1981).
- J.B. Foresman, A.E. Frisch (1996) Exploring chemistry with electronic structure methods. 2 edn. Gaussian, Inc, Wallingford, CT.
- J.S. Sreedasyam, J. Sunkari, S. Kundha, R.R. Gundapaneni, Acta Crystallogr. E. , 69, o673, (2013).
- M. Turkyilmaz, G. Uluçam, Ş. Aktaş, S.E. Okan, J. Mol. Struct., 1136, 263, (2017).
- G. Uluçam, S.E. Okan, Ş. Aktaş, G.P. Öğretmen, J. Mol. Struct., 1102, 146, (2015).
- G. Ulucam, M. Turkyilmaz, Bioinorg. Chem. Appl. , 2018, 12, (2018).
- J.S. Al-Otaibi, R.I. Al-Wabli, Spectrochim. Acta. A Mol. Biomol. Spectrosc. , 137, 7, (2015).
- Ö. Tamer, D. Avcı, Y. Atalay, J. Phys. Chem. Solids, 99, 124, (2016).
- S. Altürk, D. Avcı, Ö. Tamer, Y. Atalay, J. Mol. Struct., 1164, 28, (2018).
- S. Altürk, Ö. Tamer, D. Avcı, Y. Atalay, J. Organomet. Chem., 797, 110, (2015).
- M.T. Gabr, N.S. El-Gohary, E.R. El-Bendary, M.M. El-Kerdawy, N. Ni, M.I. Shaaban, Chin. Chem. Lett., 26, 1522, (2015).
- K.B. Gudasi, R.S. Vadavi, R.V. Shenoy, S.A. Patil, M. Nethaji, Trans. Metal. Chem., 31, 374, (2006).
- M.N. Hriday, R.K. Srivastava, V. Narayan, S. Chand, A.K. Sachan, V.K. Shukla, O. Prasad, L. Sinha, Res. J. Recent Sci. , 2, 150, (2013).


