THEORETICAL STUDY OF VARIOUS SOLVENTS EFFECT ON 5-FLUOROURACIL-VITAMIN B3 COMPLEX USING PCM METHOD
- 5-Fluorouracil,
- Vitamin B3,
- Solvent effect,
- NBO,
- QTAIM
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
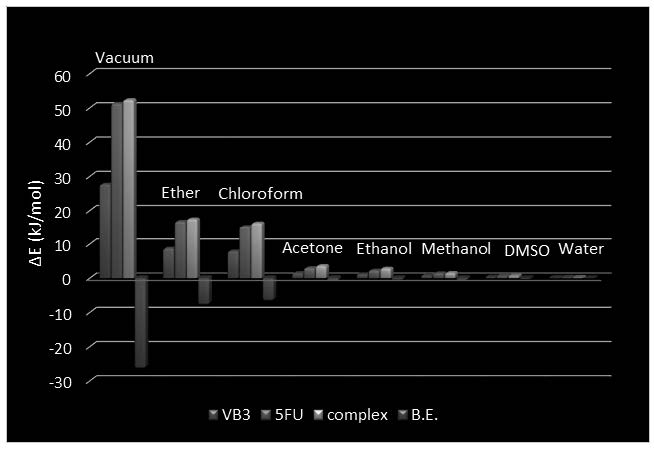
In this article, a detailed study of the solvent effects on the stability order, binding energy and hydrogen bond strength in 5-Fluorouracil-Vitamin B3 (FU–VB) complex is performed using M05-2X method with 6-311++G(d,p) basis set. Based on the average of the calculated H-bond energies, the H-bond strength of FU-VB complex in gas phase is more than solution phase. The binding energy in solution phase is also lower than the gas phase. Therefore, the stability of the studied complex increases in solution phase with respect to the gas phase. Furthermore, the topological properties of the electron density distribution are analyzed in term of the Bader Quantum Theory of “Atoms in Molecules” (QTAIM). The natural bond orbital (NBO) method is also applied to get a more precise insight into the nature of intermolecular interactions. Finally, the solvent effect on the frontier molecular orbital energies (HOMO and LUMO), chemical potential and hardness of FU–VB complex is investigated.
References
- (a) G. A. Jeffrey, D. G. Truhlar (Ed), An Introduction to Hydrogen Bonding. Oxford University Press, New York, 1997. (b) E. C. Hulme (Ed), Receptor–Ligand Interactions: A Practical Approach. Oxford University Press, Oxford, 1992.
- H. R. Masoodi, A. Ebrahimi, M. Habibi, Chem. Phys. Lett. 483, 43, (2009).
- G. R. Desiraju, T. Steiner, The Weak Hydrogen Bond: In Structural Chemistry and Biology. Oxford University Press, USA, 2001.
- D. Hadzˇi, Theoretical Treatments of Hydrogen Bonding. John Wiley & Sons, Chichester, 1997.
- P. Hobza, Z. Havlas, Chem. Rev. 100, 4253, (2000).
- W. Wang, Y. Zhang, K. Huang, Chem. Phys. Lett. 411, 439, (2005).
- A. K. Roy, A. J. Thakkar, Chem. Phys. 312, 119, (2005).
- R. Wysokinski, D. C. Biennko, D. Michalska, Th. Zeegers-Huyskens, Chem. Phys. 315, 17, (2005).
- A. Ebrahimi, H. Roohi, M. Habibi, M. Mohammadi, R. Vaziri, Chem. Phys. 322, 289, (2006).
- A. Ebrahimi, H. Roohi, M. Habibi, M. Hasannejad, Chem. Phys. 327, 368, (2006).
- A. Ebrahimi, M. Habibi, H. R. Masoodi, A. R. Gholipour, Chem. Phys. 355, 67, (2009).
- G. A. Jeffrey, W. Saenger, Hydrogen Bonding in Biology and Chemistry. Springer-Verlag, Berlin, 1991.
- C. Estarellas, A. Frontera, D. Quiñonero, P. M. Deyà, Chem. Phys. Lett. 479, 316, (2009).
- C. H. Wu, C. Chen Wu, Y. S. Ho, J. Cancer Mol. 3, 15, (2007).
- D. J. Brown, Heterocyclic Compounds: Thy Pyrimidines. Interscience, New York, 1994.
- A. F. Pozharskii, A. T. Soldatenkov, A. R. Katrizky, Heterocycles in Life and Society. John Wiley and Sons, New York, 1997.
- A. Shah, E. Nosheen, F. Zafar, S. Noman uddin, D. D. Dionysiou, A. Badshah, Z. Rehman, G. Shahzada Khan, Journal of Photochemistry and Photobiology B: Biology. 117, 269, (2012).
- J. L. Arias, Molecules. 13, 2340, (2008).
- K. Zare, F. Najafi, H. Sadegh, R. Shahryari ghoshekandi, Journal Of Nanostructure in Chemistry. 3, 71, (2013).
- M. P. Findlay, M. O. Leach, Anticancer Drugs. 5, 260, (1994).
- Institute of Medicine “Niacin”, Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington DC, The National Academies Press, 123, 1998.
- “Niacin and niacinamide (Vitamin B3)”. MedlinePlus, US National Library of Medicine, National Institutes of Health, 2016.
- S. M. Bhairi, C. Mohan, Calbiochem. 1, (2001).
- L. C. Javois, Methods in Molecular Biology. 115, 46, (1999).
- M. M. Al-Oqail, E. S. Al-Sheddi, M. A. Siddiqui, J. Musarrat, A. A. Al- Khedhairy, N. N. Farshori, Pharmacogn. Mag. 11, 598, (2015).
- S. Gayatri, C. Uma Maheswara Reddy, K. Chitra, V. Parthasarathy, Pharmacognosy Research. 7, 198, (2015).
- I. Trawczyńska, M. Wójcik, Biotechnology & Biotechnological Equipment. 29, 72, (2015).
- J. M. Baust, R. Van Buskirk, J. G. Baust, In Vitro Cell. Dev. Biol. Anim. 36, 262, (2000).
- S. Y. Lee, H. J. Park, C. Best-Popescu, S. Jang, Y. K. Park, PLoS. One. 10(12), e0145327, (2015).
- M. Frisch, G. Trucks, H. Schlegel, et al., Gaussian 03, revision D.01, Gaussian Inc., Wallingford, CT, 2004.
- S. Miertus, E. Scrocco, J. Tomasi, J. Chem. Phys. 55, 117, (1981).
- E. Espinosa, E. Molins, C. Lecomte, Chem. Phys. Lett. 285, 170, (1998).
- R. F. W. Bader, Atoms in molecules: a quantum theory. Oxford University, New York, 1990.
- F. Biegler Ko¨nig, J. Scho¨nbohm, J. Comput. Chem. 23, 1489, (2002).
- A. E. Reed, L. A. Curtiss, F. Weinhold, Chem. Rev. 88, 899, (1988).
- E. D. Glendening, A. E. Reed, J. E. Carpenter, F. Weinhold, NBO, version 3.1 (in), Gaussian, Inc., Pittsburg, CT, 2003.
- S. J. Grabowski, W. A. Sokalski, E. Dyguda, J. Leszczyn’ski, J. Phys. Chem. B. 110, 6444, (2006).
- Z. Desta, B. A. Ward, N. V. Soukhova, D. A. Flockhart, J. Pharmacol. Exp. Ther. 310, 1062, (2004).
- H. Raissi, M. Yoosefian, S. Khoshkhou, Comput. Theor. Chem. 983, 1, (2012).
- H. Raissi, F. Farzad, E. S. Nadim, M. Yoosefian, H. Farsi, A. Nowroozi, D. loghmaninejad, Int. J. Quantum Chem. 112, 1273, (2012).
- H. Raissi, M. Yoosefian, F. Mollania, F. Farzad, A. Nowroozi, D. loghmaninejad, J. Comput. Theor. Chem. 966, 299, (2011).
- M. Yoosefian, Z. Jafari Chermahini, H. Raissi, A. Mola, M. Sadeghi, J. Mol. Liq. 203, 137, (2015).
- M. Yoosefian, A. Mola, J. Mol. Liq. 209, 526, (2015).
- J. Liu, X. Xia, Y. Li, H. Wang, Z. Li, Struct. Chem. 24, 251, (2013).
- P. L. A. Popelier, J. Phys. Chem. A. 102, 1873, (1998).
- M. Noei, S. Suzangarzadeh, S. Golshani, A. Tahan, Russian J. Phys. Chem. A. 85, 993, (2011).
- P. Hobza, V. Spirko, H. L. Selzle, E. W. Schlag, J. Phys. Chem. A. 102, 2501, (1998).
- L. Hokmabady, H. Raissi, A. Khanmohammadi, Struct. Chem. 27, 487, (2016).
- I. Fleming, Frontier Orbitals: Organic Chemical Reactions. John Wiley and Sons, New York, 1976.
- J. C. Jesintha, T. S. Xavier, G. Lukose, J. I. Hubert, Spectrochimica. Acta. Part A. 85, 66, (2012).
- P. K. Chattaraj, A. Poddar, J. Phys. Chem. A. 103, 8691, (1999).
- R. G. Pearson, Chemical Hardness – Applications from Molecules to Solids. Weinheim: VCH-Wiley, 1997.
- R. G. Parr, L. von Szentpaly, S. Liu, J. Am. Chem. Soc. 121, 1922, (1999).
- K. D. Sen, C. K. Jorgensen, Electronegativity, Structure and Bonding. Springer Verlag, New York, 1987.
- T. Koopmans, Phys. 1, 104, (1933).
- L. R. Domingo, M. Ríos-Gutiérrez, P. Pérez, Molecules. 21, 748, (2016).
- L. R. Domingo, M. J. Aurell, P. Pérez, R. Contreras, Tetrahedron. 58, 4417, (2002).
- J. S. Murray, K. Sen, Molecular Electrostatic Potentials, Concepts and Applications. Elsevier, Amsterdam, 1996.


