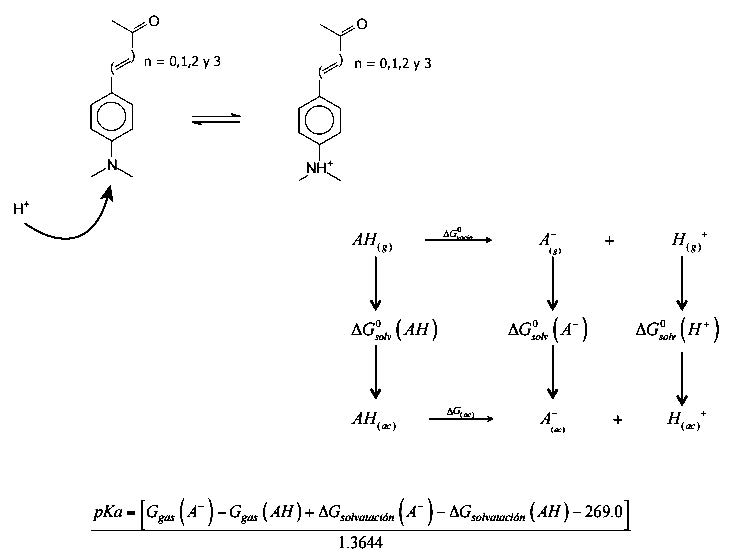
THERMODYNAMIC CYCLE FOR CALCULATING AB-INITIO pKa VALUES OF TYPE (Me)2-N-Phenyl-(HC=CH)n-CHO (n = 0, 1, 2 and 3) MOLECULAR SYSTEMS
- Thermodynamic cycle,
- PKa,
- Proton affinity
Copyright (c) 2017 Hernández T. Carlos

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Based on a thermodynamic cycle the pKa in aqueous phase of the series of molecules of the type (Me)2-N-Phenyl-(HC=CH)n-CHO with n = 0, 1, 2 and 3 have been determined. To that end the SM5.4 solvation model has been considered. The calculated pKa have been compared with the pKa measured experimentally. A study has also been made of the proton affinity in the gas phase and in aqueous phase. The calculation scheme agrees favorably, in a qualitative manner, considering favorably the molecular and solute-solvent interaction characteristics that determine the free energy that governs the acid-base properties of the molecules in the series.
References
- Avdeef, A. (2012). Absorption and drug development: solubility, permeability, and charge state. John Wiley & Sons.
- Andricopulo, A. D., & Montanari, C. A. (2005). Mini reviews in medicinal chemistry, 5(6), 585-593.
- Jamzad, S., & Fassihi, R. (2006). Aaps Pharmscitech, 7(2), E17-E22.
- Prevedouros, K., Cousins, I. T., Buck, R. C., & Korzeniowski, S. H. (2006). Environmental Science & Technology, 40(1), 32-44.
- Manallack, D. T. (2007). Perspectives in medicinal chemistry, 1, 25.
- S.E. Blanco, M.C. Almandoz and F.H. Ferretti, Spectrochim. Acta A 61, (2005).
- B.M. Schmidt and E.-W. Knapp, Chemphyschem 5, (2004).
- Yao Fu, Lei Liu, Rui-Qiong Li, Rui Liu and Qing-Xiang Guo, J. Am. Chem. Soc. 26, (2004).
- (a)M.D. Liptak, K.C. Gross, P.G. Seybold, S. Feldgus and G.C. Shields, J. Am. Chem. Soc. 124 (2002), (b)M.D. Liptak and G.C. Shields, J. Am. Chem. Soc. 123 (2001). (c)K. Murlowska and N. Sadlej-Sosnowska, J. Phys. Chem. A 109 (2005).
- D. Jacquemin, E.A. Perpete, I. Ciofini and C. Adamo, J. Phys. Chem. A 112 (2008).
- H. Lu, X. Chen and C.-G. Zhan, J. Phys. Chem. B 111 (2007).
- X.-Q. Zhu, C.-H. Wang, H. Liang and J.-P. Cheng, J. Org. Chem. 72 (2007).
- I.E. Charif, S.M. Mekelleche, D. Villemin and N. Mora- Diez, THEOCHEM 818 (2007).
- R. F. Cookson Chem. Rev., 74 (1), (1974).
- SPARTAN ‘06 Quantum Mechanics Program: (PC/x86) Release 129v3 (Wavefunction Inc. Irvine, CA).
- Theoretica Chimica Acta, 98, (1997).
- Cox, B. G. (2013). Acids and bases: solvent effects on acid-base strength. Oxford University Press.
- Śmiechowski, M. (2010). Chemical Physics Letters, 501(1), 123-129.
- S. Miertus, E. Scrocco and J. Tomasi, Chem. Phys. 55 (1981).
- V. Barone and M. Cossi, J. Chem. Phys. A 102 (1998).
- Candee C. Chambers, Gregory D. Hawkins, Christopher J. Cramer Donald G. Truhlar J. Phys. Chem. Vol 100, (1996).
- Hawkins, G. D., Cramer, C. J., & Truhlar, D. G. (1996). Journal of Physical Chemistry, 100(51), 19824-19839.
- Hernández T. Carlos, Congreso Latinoamericano de Química, Salvador de Bahía, Brasil, 2005.
- Handbook.of.Chemistry.and.Physics.v2010.ISO-HS.
- Sharon G. Lias, Jo-Anne A. Jacksonla, Harold Argentarlb, Joel F. Liebman; J. Org. Chem. 50, (1985).-
- Walder Ray L. and Franklin J.L.; International Journal of Mass Spectrometry and Ion Physics, 36 (1960).


