MESO AND RACEMIC 1,1-BIS (BENZYLSULFINYL)-2-ETHYLBUTANE. A b-DISULFOXIDE PRO-LIGAND WITH STERIC RESTRICTIONS
Copyright (c) 2017 M. Zárraga O, Y. Moreno N., C. Franco O.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
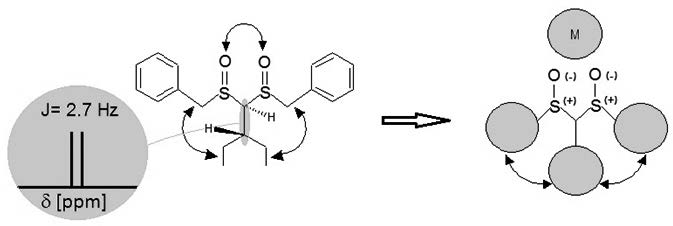
Coordination chemistry of disulfoxides is of interest in inorganic chemistry given the well-known metal coordination through oxygen or sulfur atoms.1 Ethylene and propylene bridged disulfoxide pro-ligands, coordinate to metals, forming coordinated and chelated complexes, demonstrating its flexibility in the formation of such complexes.2 Some of these compounds have demonstrated interesting properties in medicinal chemistry3 and asymmetric catalysis4 and their synthesis is of importance for bioorganic studies. The presence of two stereogenic centers allows the existence of the meso and racemic forms in disulfoxides. Previous studies on b-disulfoxides5 in our laboratories have led to the synthesis and spectroscopic study (1H-NMR, FT-IR) of both diastereomeric forms. In this work we report the synthesis and structural analysis of meso-(2)- and racemic-(3)-1,1-bis(benzylsulfinyl)-2-ethylbutane (Scheme1), with high steric requirement around both sulfinyl groups when a branched aliphatic carbon chain is introduced in the a-carbon. An increase in selectivity for small radius metal ions was achieved by compression of the pro-ligand coordination radius. These kind of compounds have not been previously studied.
References
- a) Corey, E. J.; Chaykovsky, M. J. Amer. Chem. Soc. 1964, 86, 1345. b) Madan, S. K.; Hull, C. M.; Herman, L. J. J. Chem. Soc. 1968, 491-495. c) Zipp, A. P.; Madan, S. K. Inorg. Chim. Acta. 1977, 22, 49-53.
- a) Li, Jian-Rong.; Zhang, Ruo-Hua.; Bu, Xian-He. Aust. J. Chem. 2006, 59, 315-319. b) Chen, Wei.; Du, Miao.; Zhang, Ruo-Hua.; Bu, Xian-He. Chinese J. Struct. Chem. 2002, 5, 277-279. c) Geremia, S.; Calligaris, M.; Mestromi, S. Inorg. Chim. Acta. 1999, 292, 144-146.
- Kagan, H. B.; Ronan, B. Reviews on Heteroatom Chemistry. 1992, 7, 92- 117.
- a) Gelle´rt Sipos, Emma E. Drinkel, Reto Dorta Chem. Soc. Rev., 2015, 44, 3834 b) Fernández, I.; Araújo, C. S.; Alcudia, F.; Khiar, N. Phosphorous, Sulfur and Silicon, 2005, 180, 1509-1510.
- a) Franco, O. C.; Zárraga, O. M.; Zunza, E. H. Bol. Soc. Chil. Quim. 1995, 40, 319-324. b) Zárraga, O. M.; Zunza, E. H.; Franco, O. C.; Pérez, H. C.; Bol. Soc. Chil. Quim. 1996, 41, 307-309.
- Gaussian 09, Revision A.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
- Calligaris, M.; Melchior, A.; Geremia, S. Inorg. Chim. Acta. 2001, 323, 89-95.


