SYNTHESIS, ANTI-PHYTOPATHOGENIC AND DPPH RADICAL SCAVENGING ACTIVITIES OF C-PRENYLATED ACETOPHENONES AND BENZALDEHYDES
- C-prenylation,
- Prenylated acetophenone,
- Prenylated benzaldehyde,
- Electrophilic aromatic substitution,
- Anti-phytopathogenic
- DPPH radical scav¬enging activity ...More
Copyright (c) 2017 Mauricio E. Osorio, Karol A. Quiroz, Marcela A. Carvajal, Alejandra P. Vergara, Elizabeth Y. Sánchez, Cesar E. González, Karen S. Catalán

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
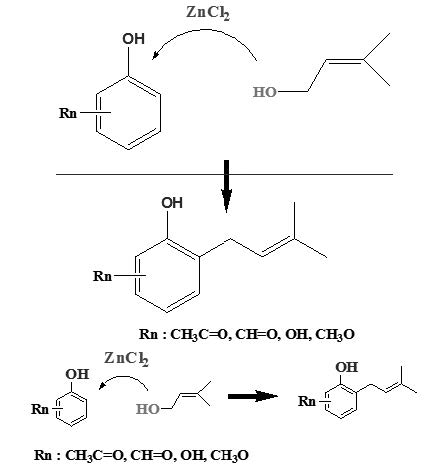
The syntheses of six prenylated acetophenone and benzaldehyde derivatives and their anti-phytopathogenic and antioxidant activities are reported. These compounds were obtained by electrophilic aromatic substitution (SEAr) of the corresponding arenes and 3-methyl-2-buten-1-ol using ZnCl2 as a Lewis acid catalyst in ethyl acetate. Reasonable to good yields were obtained based on unrecovered aromatic starting material (45-73%). All the synthesized compounds were evaluated against phytopathogenic gram-negative bacteria Agrobacterium tumefaciens, Pseudomonas syringae and Erwinia carotovora and plant fungal pathogens Botrytis cinerea, Phytophthora cinnamomi and Gibberella fujikuroi. The antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay and expressed as half maximal inhibitory concentration (IC50) values in μM concentrations. The antioxidant activity went from 27.20 μM to >100 μM. Compound 11 showed statistically significant inhibition of the growth of Botrytis cinerea, and compounds 13 and 15 showed statistically significant inhibition of the growth of Phytophthora cinnamomi, with respect to negative control fungal growth. All six compounds showed bacteriostatic effects against gram-negative plant pathogenic bacteria with IC50 values between 250 and <3.9 μM.
References
- N. Edayadulla, P. A. Ramesh, Nat. Prod. Commun. 7, 1325 (2012).
- M.E. Sánchez-Mendoza, J. Rodríguez-Silverio, J.F. Rivero-Cruz, H.I. Rocha-González, J.B. Pineda-Farías, J. Arrieta, Fitoterapia 87, 11 (2013).
- B. Sontag, N. Arnold, W. Steglich, T. Anke, J. Nat. Prod. 62, 1425 (1999).
- R.K. Mayaka, M.K. Langat, J.O. Omolo, P.K. Cheplogoi, Planta Med. 78, 383 (2012).
- E. Jiménez, F. Dorta, C. Medina, A. Ramírez, I. Ramírez, H. Peña-Cortés, Mar. Drugs 9, 739 (2011).
- W. El Khoury, K. Makkouk, J. Plant Pathol., 92 (4, Supplement), S4.35 (2010).
- B. Williamson, B. Tudzynski, P. Tudzynski, J.A.L. Van Kan, Mol. Plant Pathol. 8, 561 (2007).
- E.G. Wulff, J.L. Sørensen, M. Lübeck, K.F. Nielsen, U. Thrane, J. Jan Torp, Environ. Microbiol. 12, 649 (2010).
- G. Sepúlveda-Chavera, R. Salvatierra-Martínez, C. Bilbao-Apata, P. Sepúlveda-Ramírez, M. Allende-Castro, J. Alache-González, IDESIA (Chile), 31, 41 (2013).
- L. Gallo, F. Siverio, A.-M. Rodríguez-Pérez, Ann. Appl. Biol. 150, 65 (2007).
- Ozone Secretariat United Nations Environment Programme (UNEP), Handbook for the Montreal Protocol on Substances that Deplete the Ozone Layer, 8th ed.; Secretariat for The Vienna Convention for the Protection of the Ozone Layer & The Montreal Protocol on Substances that Deplete the Ozone Layer: Nairobi, Kenya, 2009; pp. 291-292.
- CODEX ALIMENTARIUS International Food Standards. http://www. codexalimentarius.org/about-codex/en/. (accessed on 26 January 2016).
- M. Guo, A. Block, C.D. Bryan, D.F. Becker, J.R. Alfano, J. Bacteriol. 194, 5054 (2012).
- R. Prajapat, A. Marwal, P.N. Jha, Int. J. Curr. Microbiol. App. Sci. 2, 83 (2013).
- L.W. Moore, J.W. Pscheidt, Diseases Caused by Pseudomonas syringae. http://pnwhandbooks.org/plantdisease/node/1814/print. (accessed on 26 January 2016).
- C. Hoarau, T.R.R. Pettus, Synlett 1, 127 (2003).
- O Talhi, A.M.S. Silva, Curr. Org. Chem. 17, 1067 (2013).
- S.R. Ravada, L.R. Emani, M.R. Garaga, B. Meka, T. Golakoti, Am. J. Infect. Dis. 5, 83 (2009).
- S.N. Fedorov, O.S. Radchenko, L.K. Shubina, N.N. Balaneva, A.M. Bode, V.A. Stonik, Z. Dong, Pharm. Res. 23, 70 (2006).
- Z. Wang, Y. Cao, S. Paudel, G. Yoon, S.H. Cheon, Arch. Pharm. Res. 36, 1432 (2013).
- J.-J. Helesbeux, O. Duval, D. Guilet, D. Séraphin, D. Rondeau, P. Richomme, Tetrahedron 59, 5091 (2003).
- M.P. Neves, R.T. Lima, K. Choosang, P. Pakkong, M. de S.J. Nascimento, M.H. Vasconcelos, M. Pinto, A.M.S. Silva, Cidade, H. Chem. Biodivers. 9, 1133 (2012).
- X. Dong, Y. Wang, T. Liu, P. Wu, J. Gao, J. Xu, B. Yang, Y. Hu, Molecules 16, 8257 (2011).
- A.C. Jain, R.C. Gupta, P.D. Sarpal, Tetrahedron 34, 3563 (1978).
- V.P. Pathak, R.N. Khanna, Bull. Chem. Soc. Jpn. 55, 2264 (1982).
- W. Reininger, A. Hartl, Method of acylation of phloroglucinol. US patent 4,053,517, filed 5 May 1976, issued 11 October 1977.
- D. Ahmed, M.M. Khan, R. Saeed, Antioxidants 4, 394 (2015).
- T.P.T. Cushnie, A.J. Lamb, Int. J. Antimicrob. Agents 38, 99 (2011).
- M. Daglia, Curr. Opin. Biotechnol. 23, 174 (2012).
- H. Naeimi, A. Amini, M. Moradian, Org. Chem. Front. 1, 415 (2014).
- E. Bálint, O. Kovács, L. Drahos, Keglevich, G. Lett. Org. Chem. 10, 330 (2013).
- M. Vandewalle, Bol. Soc. Chim. Belg. 70, 163 (1961).
- S. Alam, J. Miah, A. Islam, J. Biol. Sci. 4, 527 (2004).
- L.Y. Zhao, Y.L. Li, Chin. Chem. Lett. 5, 1009 (1994).
- M. Osorio, J. Aravena, A. Vergara, L. Taborga, E. Baeza, K. Catalán, C. González, M. Carvajal, H. Carrasco, L. Espinoza, Molecules 17, 556 (2012).
- M.D. Cole, Biochem. Syst. Ecol. 22, 837 (1994).
- I.C. Zampini, M.A. Vattuone, M.I. Isla, J. Ethnopharmacol. 102, 450 (2005).
- P. Cos, A.J. Vlietinck, D.V. Berghe, L. Maes, J. Ethnopharmacol. 106, 290 (2006).
- P.S. McManus, V.O. Stockwell, G.W. Sundin, A.L. Jones, Ann. Rev. Phytopathol. 40, 443 (2002).
- S. Riggoti, K. Gindro, H. Richter, O. Viret, FEMS Microbiol. Lett. 209, 169 (2002).
- T. Bekker, N. Labuschagne, C. Kaiser, S. Afr. Avocado Grow. Assoc. Yearb 28, 60 (2005).
- M. Horta, N. Sousa, A.C. Coelho, D. Neves, A. Cravador, Physiol. Mol. Plant Pathol. 73, 48 (2008).
- M. Azor, J. Gené, J. Cano, D.A. Sutton, A.W. Fothergill, M.G. Rinaldi, J. Guarro, Antimicrob. Agents Chemother. 52, 2228 (2008).
- R. Salzman, I. Tikhonova, B. Bordelon, P. Hasegawa, R. Bressan, Plant Physiol. 117, 465 (1998).
- S.I. Kirubakaran, S.M. Begum, K. Ulaganathan, N. Sakthivel, Plant Physiol. Biochem. 46, 918 (2008).
- G. Zhu, F. Huang, L. Feng, B. Qin, Y. Yang, Y. Hen, X. Lu, Agric. Sci. China 7, 831 (2008).
- M. Meepagala, G. Sturtz, G. Wedge, J. Agric. Food Chem. 50, 6989 (2002).
- H. Alizadeh, A. Sharifi-Tehrani, G. Hedjaroude, Agric. Appl. Biol. Sci. 72, 795 (2007).
- W. Steck, Can. J. Chem. 49, 1197 (1971).


