- nickeloxime,
- dehalogenation,
- electrocatalysis
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
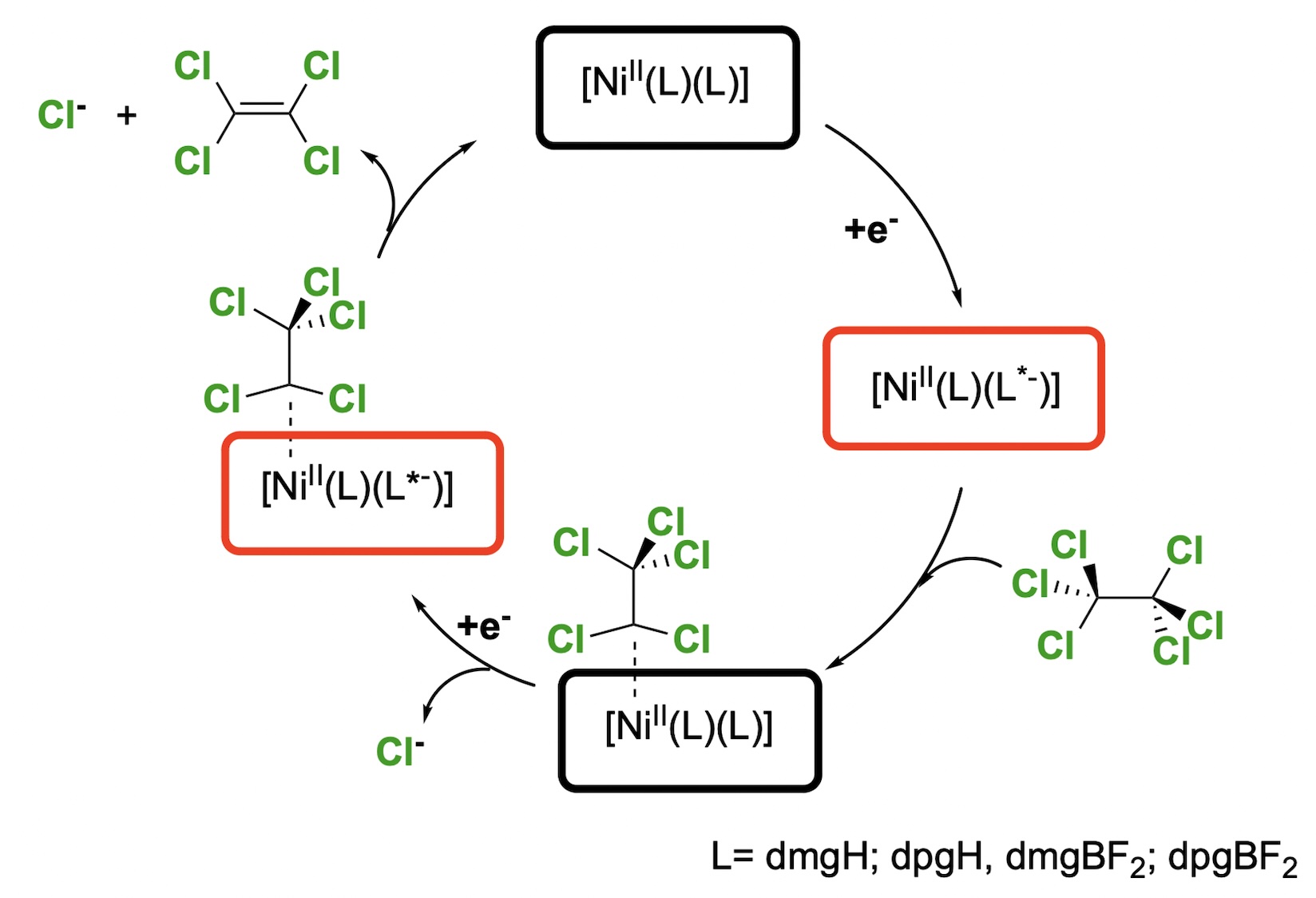
The electrochemical degradation of hexachloroethane using bis(dimethylglyoximato)nickel(II), [Ni(dmgH)2]; bis(diphenylglyoximato)nickel(II), [Ni(dpgH)2]; difluoroborylbis(dimethylglyoximato)nickel(II), [Ni(dmgBF2)2]; and difluoroborylbis(diphenylglyoximato)nickel(II), [Ni(dpgBF2)2], is described. To achieve the degradation the nickel complexes were reduced by using electrochemical means. The chemical catalysis was studied using cyclic voltammetry by monitoring currents increases as the concentration of hexachloroethane was increase in solution. The rate constant of the dehalogenation process was estimated using foot-of-the-wave analysis (FOWA), obtaining: [Ni(dmgH)2] 6.58 x 104; [Ni(dpgH)2] 6.74 x 104; [Ni(dmgBF2)2] 7.06 x 104 and [Ni(dpgBF2)2] 1.08 x 104.

References
- E. Abbasi, Z. Yazdani, S. Daliri and M. D. Moemenbellah-Fard, Parasite Epidemiol Control (2023), 22, e00310.
- G. W. Gribble, J. Chem. Educ. (2004), 81, 1441.
- Q. Qing Li, A. Loganath, Y. Seng Chong, J. Tan and J. Philip Obbard, J. Toxicol. Environ. Health. A. (2006), 69, 1987–2005.
- M. C. Keifer and J. Firestone, J. Agromedicine. (2007), 12, 17–25.
- R. Jayaraj, P. Megha and P. Sreedev, Interdiscip. Toxicol. (2016), 9, 90–100.
- U.S. Department of Health and Human Services, National Toxicology Program (2011), 53.
- F. Aulenta, M. Majone, P. Verbo and V. Tandoi, Biodegradation (2002), 13, 411–424.
- C. Ma and Y. Wu, Environmental Geology (2008), 55, 47–54.
- M. A. Esteruelas, J. Herrero and M. Oliván, Organometallics (2004), 23, 3891–3897.
- M. Bressan, N. d’Alessandro, L. Liberatore and A. Morvillo, Coord. Chem. Rev. (1999), 185–186, 385–402.
- M. E. Cucullu, S. P. Nolan, T. R. Belderrain and R. H. Grubbs, Organometallics (1999), 18, 1299–1304.
- L. Szatkowski and M. B. Hall, Dalton Trans. (2016), 45, 16869–16877.
- K. C. Pillai, G. Muthuraman and I.-S. Moon, Electrochim. Acta (2017), 232, 570–580.
- Y. Kashiwagi, C. Kikuchi and J. Anzai, J. Electroanal. Chem. (2002), 518, 51–55.
- K. Mochizuki and M. Suzuki, Inorg. Chem. Commun. (2011), 14, 902–905.
- C. A. Rettenmeier, J. Wenz, H. Wadepohl and L. H. Gade, Inorg. Chem. (2016), 55, 8214–8224.
- S. Pizarro, M. Araya and A. Delgadillo, Polyhedron (2017), 141, 94–99.
- C. Costentin and J.-M. Savéant, ChemElectroChem. (2014), 1, 1226–1236.
- I. F. Bruce-Smith, B. A. Zakharov, J. Stare, E. V. Boldyreva and C. R. Pulham, J. Phys. Chem. C (2014), 118, 24705–24713.
- M. Cowie, A. Gleizes, G. W. Grynkewich, D. W. Kalina, M. S. McClure, R. P. Scaringe, R. C. Teitelbaum, S. L. Ruby and J. A. Ibers, J. Am. Chem. Soc. (1979), 101, 2921–2936.
- F. S. Stephens, R. S. Vagg and E. C. Watton, Inorg. Chim. Acta (1981), 47, 97–104.
- S. Pizarro, M. Araya and A. Delgadillo, Polyhedron (2018), 141, 94–99.
- E. V. Patterson, C. J. Cramer and D. G. Truhlar, J. Am. Chem. Soc. (2001), 123, 2025–2031.
- O. Pantani, E. Anxolabéhère-Mallart, A. Aukauloo and P. Millet, Electrochem. Commun. (2007), 9, 54–58.
- N. Elgrishi, M. B. Chambers and M. Fontecave, Chem. Sci. (2015), 6, 2522–2531.
- V. Artero and J.-M. Saveant, Energy Environ. Sci. (2014), 7, 3808–3814.
- C. Costentin and J.-M. Savéant, J. Am. Chem. Soc. (2017), 139, 8245–8250.
- B. Huang, A. A. Isse, C. Durante, C. Wei and A. Gennaro, Electrochim. Acta (2012), 70, 50–61.
- D. Lexa, J. M. Saveant, H. J. Schaefer, Su Khac Binh, B. Vering and D. L. Wang, J. Am. Chem. Soc. (1990), 112, 6162–6177.
- Q. Lin, Y. Fu, P. Liu and T. Diao, J. Am. Chem. Soc. (2021), 143, 14196–14206.

