A COMPUTATIONAL STUDY OF STEVIOL AND ITS SUGGESTED ANTICANCER ACTIVITY. A DFT AND DOCKING STUDY.

- Steviol,
- BCL-2,
- antiapoptotic,
- acceptor-donor,
- DFT
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
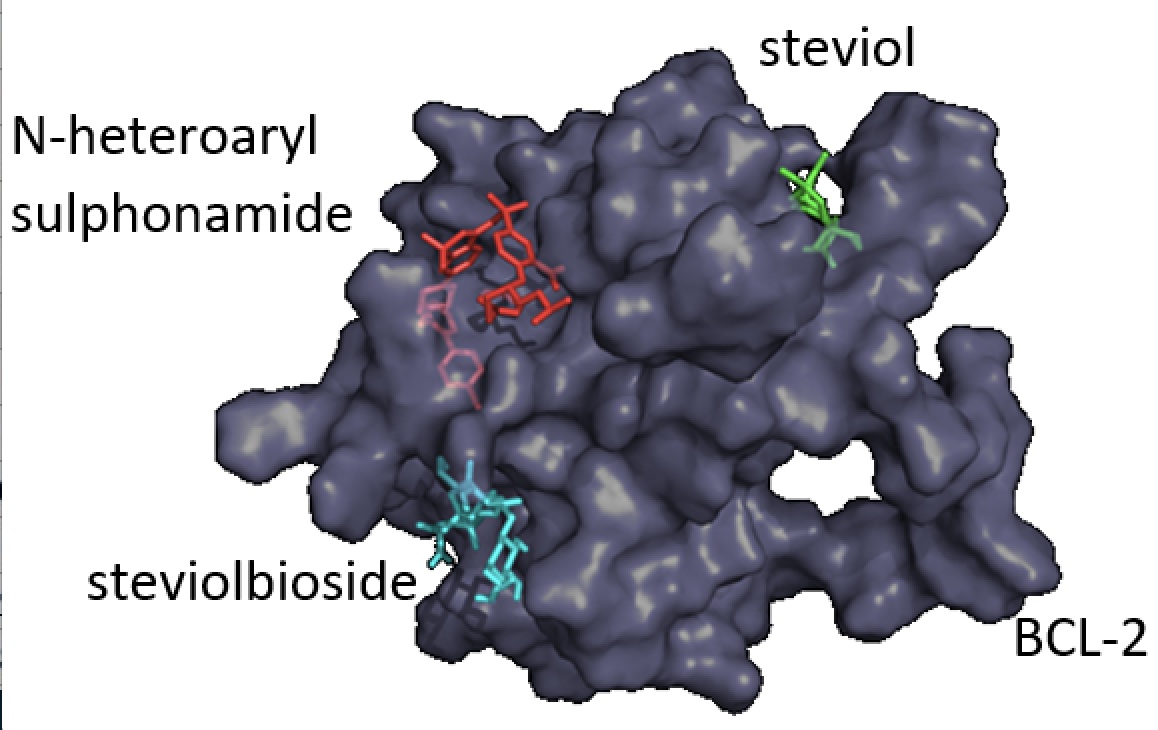
In the present, study we analyzed the electronic properties of Steviol, the Stevia rebaudiana metabolite, and its interaction with antiapoptotic protein BCL-2. The ionization potential and electrophilicity index values were evaluated in the framework of the DFT, and these values suggest that Steviol may form ligand-receptor interactions. Also, the bond dissociation energy and the electrostatic potential distribution of Steviol reveal its antioxidant behavior. Docking studies were performed to evaluate the feasibility of this molecule to interact with antiapoptotic protein BCL-2. However, no hydrogen bonds were found in the pocket site, instead six interactions, including alkyl and π-alkyl type were formed, suggesting that the possible most feasible mechanism for anticancer activity would be through free radicals scavenging.
References
- J. Chen, Y. Xia, X. Sui, Q. Peng, T. Zhang, J. Li, J. Zhang, Oncotarget (2018), 9, 26299–26308.
- M. Reddy Mallu, S. Vemula, R. K. Kante, . Pharm. Sci. Res. (2019), 11, 2016–2018. https://www.jpsr.pharmainfo.in/Documents/Volumes/vol11issue05/jpsr11051962.pdf. Accessed April 26, 2021.
- E. Gupta, S. Kaushik, S. Purwar, R. Sharma, A. Balapure, S. Sundaram, Pharmacogn. Mag. (2017), 13, 345.
- S. Ghanta, A. Banerjee, A. Poddar, S. Chattopadhyay, J. Agric. Food Chem. (2007), 55, 10962–10967.
- C. Panagiotou, C. Mihailidou, G. Brauhli, O. Katsarou, P. Moutsatsou, Mol. Cell. Endocrinol. (2018), 460, 189–199.
- T. Pasqualli, P. E. E. Chaves, c, L. da V. Pereira, É. A. Serpa, L. F. S. D. Oliveira, M. M. Machado, Immunopharmacol. Immunotoxicol. (2020), 42, 504–508.
- D. J. Brusick, Food Chem. Toxicol. (2008) 46, S83-91.
- A. M. Petros, A. Medek, D. G. Nettesheim, D. H. Kim, H. S. Yoon, K. Swift, E. D. Matayoshi, T. Oltersdorf, S. W. Fesik, Proc. Natl. Acad. Sci. U. S. A. (2001), 98, 3012–3017.
- S. Kim, J. Chen, T. Cheng, A. Gindulyte, J. He, S. He, Q. Li, B. A. Shoemaker, P. A. Thiessen, B. Yu, L. Zaslavsky, J. Zhang, E. E. Bolton, Nucleic Acids Res. (2019), 47, D1102–D1109.
- R. Dennington, T. Keith, J. Millam, Gaussview, Version 5., 2016.
- Y. Zhao, D. G. Truhlar, Theor. Chem. Acc. (2008), 120, 215–241.
- S. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. (2011), 32, 1456–1465.
- S. Grimme, J. Comput. Chem. (2006), 27, 1787–1799.
- J. Da Chai, M. Head-Gordon, J. Chem. Phys. (2008), 128, 84106.
- J. Da Chai, M. Head-Gordon, Phys. Chem. Chem. Phys. (2008), 10, 6615–6620.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V Ortiz, A. F. Izmaylov, J. L. Sonnenberg, Williams, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, D. J. Fox, (2016).
- A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. B (2009), 113, 6378–6396.
- J. Tomasi, B. Mennucci, R. Cammi, Chem. Rev. (2005), 105, 2999–3093.
- R. G. Pearson, Inorganica Chim. Acta (1992), 198–200, 781–786.
- R. G. Parr, L. V. Szentpály, S. Liu, J. Am. Chem. Soc. (1999), 121, 1922–1924.
- D. S. Wishart, Y. D. Feunang, A. C. Guo, E. J. Lo, A. Marcu, J. R. Grant, T. Sajed, D. Johnson, C. Li, Z. Sayeeda, N. Assempour, I. Iynkkaran, Y. Liu, A. MacIejewski, N. Gale, A. Wilson, L. Chin, R. Cummings, Di. Le, A. Pon, C. Knox, M. Wilson, Nucleic Acids Res. (2018), 46, D1074–D1082.
- H. M. Berman, T. Battistuz, T. N. Bhat, W. F. Bluhm, P. E. Bourne, K. Burkhardt, Z. Feng, G. L. Gilliland, L. Iype, S. Jain, P. Fagan, J. Marvin, D. Padilla, V. Ravichandran, B. Schneider, N. Thanki, H. Weissig, J. D. Westbrook, C. Zardecki, Acta Crystallogr. Sect. D Biol. Crystallogr. (2002), 28, 235–242.
- G. Morris, R. Huey, W. Linkstrom, M. Sanner, R. Belew, D. Goodsell, Olson, J. Comput. Chem. (2009), 16, 2785–2791.
- L. Schrödinger, Thomas Hold. (2015).
- Y. Liu, M. Grimm, W. tao Dai, M. chun Hou, Z. X. Xiao, Y. Cao, Acta Pharmacol. Sin. (2020), 41, 138–144.
- O. Trott, A. J. Olson, J. Comput. Chem. (2010), 31, 455–461.
- R. A. Laskowski, M. B. Swindells, J. Chem. Inf. Model. (2011), 51, 2778–2786.
- M. C. Flores, E. A. Márquez, J. R. Mora, Med. Chem. Res. (2018), 27, 844–856.
- E. Cortes, J. R. Mora, E. Márquez, Crystals (2020), 10, 692.
- B. B. Touré, K. Miller-Moslin, N. Yusuff, L. Perez, M. Doré, C. Joud, W. Michael, L. Dipietro, S. Van Der Plas, M. McEwan, F. Lenoir, M. Hoe, R. Karki, C. Springer, J. Sullivan, K. Levine, C. Fiorilla, X. Xie, R. Kulathila, K. Herlihy, D. Porter, M. Visser, ACS Med. Chem. Lett. (2013), 4, 186–190.
- R. W. Birkinshaw, J. nan Gong, C. S. Luo, D. Lio, C. A. White, M. A. Anderson, P. Blombery, G. Lessene, I. J. Majewski, R. Thijssen, A. W. Roberts, D. C. S. Huang, P. M. Colman, P. E. Czabotar, Nat. Commun. (2019), 10, 1–10.
- K. Palanichamy, A. Joshi, T. Mehmetoglu-Gurbuz, M. F. Bravo, M. A. Shlain, F. Schiro, Y. Naeem, H. Garg, A. B. Braunschweig, J. Med. Chem. (2019), 62, 4110–4119.
- O. Francesconi, C. Nativi, G. Gabrielli, I. De Simone, S. Noppen, J. Balzarini, S. Liekens, S. Roelens, Chem. - A Eur. J. (2015), 21, 10089–10093.

