SYNTHESIS, SPECTRAL ANALYSIS AND ANTIBACTERIAL EVALUATION OF 5-SUBSTITUTED-1,3,4- OXADIAZOL-2-YL 4-(4-METHYLPIPERIDIN-1-YLSULFONYL)BENZYL SULFIDES
- 1,
- 3,
- 4-oxadiazole,
- 4-methylpiperidine,
- Antibacterial activity
- Sulfonamide,
- 1H-NMR,
- EI-MS ...More
Copyright (c) 2017 Aziz Ur Rehman, Samreen Ahtzaz, Muhammad Athar Abbasi, Sabahat Zahra Siddiqui, Shahid Rasool, Irshad Ahmad

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
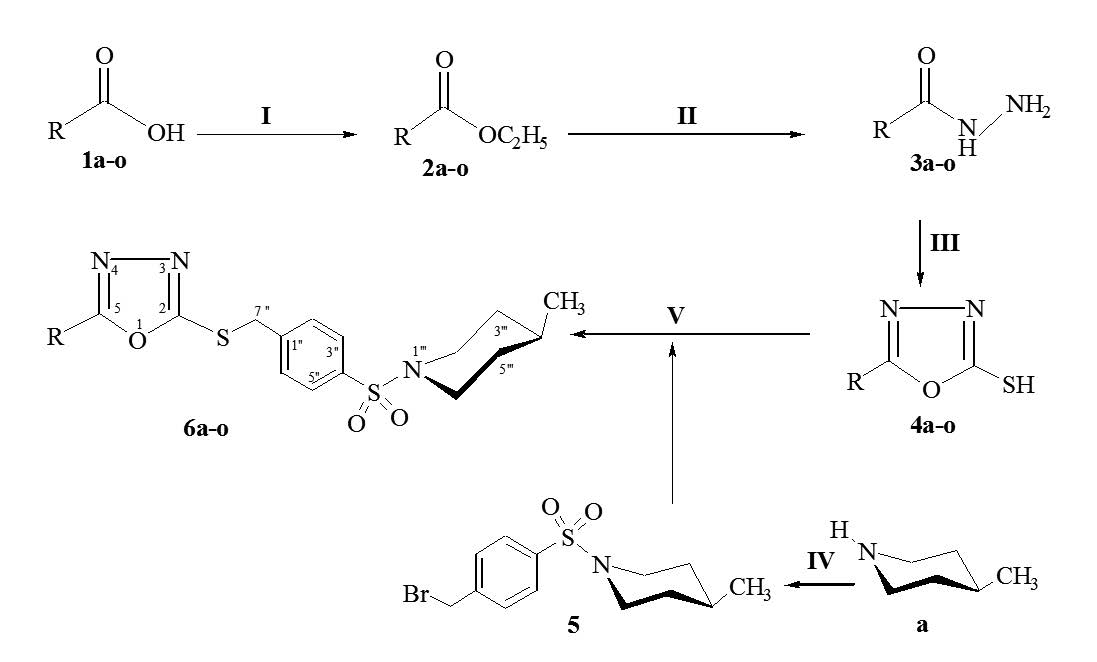
Owing to valuable biological activities of 1,3,4-oxadiazole, sulfamoyl and piperidine functionalities, some new 1-(4-{[(5-substituted-1,3,4-oxadiazol-2-yl) thio]methyl}benzene sulfonyl)-4-methylpiperidine (6a-o) derivatives have been introduced. The target molecules were synthesized from different aralkyl/aryl carboxylic acids, 1a-o, through a series of steps. First the compounds, 1a-o, were converted to heterocyclic 1,3,4-oxadiazole nucleophiles, 4a-o. Second an electrophile as 1-(4-bromomethylbenzenesulfonyl)-4-methylpiperidine (5) was synthesized from 4-methylpiperidine. Finally the target compounds, 6a-o, were prepared by reacting 4a-o with 5 in DMF and LiH. The final compounds were structurally elucidated by spectral data of IR, 1H-NMR and EI-MS. All the compounds were screened for their antibacterial evaluation and found to exhibit valuable results.
References
- P. C. Sharma, S. Jain, Acta Pharma. Sci. 50, 35 (2008).
- P. C. Sharma, S. Jain, Acta Pol. Pharm. Drug Res. 65, 551 (2008).
- Y. Q. Ge, J. Jia, T. Wang, H. W. Sun, G. Y. Duan, J. W. Wang, Spectrochim. Acta Mol. Biomol. Spectrosc. 123, 336 (2014).
- K. Zhang, P. Wang, L. N. Xuan, X. Y. Fu, F. Jing, S. Li, Y. M. Liu, B. Q. Chen, Bioorg. Med. Chem. Lett. 24, 5154 (2014).
- P. Li, L. Shi, M. N. Gao, X. Yang, W. Xue, L. H. Jin, D. Y. Hu, B. A. Song, Bioorg. Med. Chem. Lett. 25, 481 (2014).
- M. M. G. El-Din, M. I. El-Gamal, M. S. Abdel-Maksoud, K. H. Yoo, C. H. Oh, Bioorg. Med. Chem. Lett. 25, 1692 (2015).
- M. M. G. El-Din, M. I. El-Gamal, M. S. Abdel-Maksoud, K. H. Yoo, C. H. Oh, Eur. J. Med. Chem. 90, 45 (2014).
- A. A. Othman, M. Kihel, S. Amara, Arabian J. Chem. http://dx.doi. org/10.1016/j.arabjc.2014.09.003 (2014).
- J. Lian, Z. Qiang, M. Li, J. R. Bolton, J. Qu, Water Res. 75, 43 (2015).
- T. A. Kung, C. W. Tsai, B. C. Ku, W. H. Wang, Food Chem. 175, 189 (2015).
- M. Farahi, B. Karami, H. M. Tanuraghaj, Tetrahedron Lett. 56, 1833 (2015).
- S. Konda, S. Raparthi, K. Bhaskar, R. K. Munaganti, V. Guguloth, L. Nagarapu, D. M. Akkewar, Bioorg. Med. Chem. Lett. 25, 1643 (2015).
- C. C. Yang, C. L. Huang, T. C. Cheng, H. T. Lai, Biodegradation http:// dx.doi.org/10.1016/j.ibiod.2015.01.015 (2015).
- M. M. Ghorab, F. A. Ragab, H. I. Heiba, M. G. El-Gazzar, S. S. Zahran, Eur. J. Med. Chem. 92, 682 (2015).
- S. R. Khobare, V. S. Gajare, N. Rajana, R. Datrika, K. S. Reddy, U. K. S. Kumar, V. Siddaiah, Tetrahedron Lett. http://dx.doi.org/10.1016/j. tetlet.2015.02.042 (2015).
- V. D. Vitnik, Z. J. Vitnik, Spectrochim. Acta Mol. Biomol. Spectrosc. 138, 1 (2015).
- S. D. Castro, M. J. Camarasa, J. Balzarini, S. Velazquez, Eur. J. Med. Chem. 83, 174 (2014).
- Aziz-ur-Rehman, A. Fatima, N. Abbas, M. A. Abbasi, K. M. Khan, M. Ashraf, I. Ahmad, S. A. Ejaz, Pak. J. Pharm. Sci. 26, 345 (2013).
- Aziz-ur-Rehman, A. Fatima, M. A. Abbasi, S. Rasool, A. Malik, M. Ashraf, I. Ahmad, S. A. Ejaz, J. Saudi Chem. Soc. Doi:http://dx.doi. org/10.1016/j.jscs.2013.02.006 (2013).
- M. Kaspady, V. K. Narayanaswamy, M. Raju, G. K. Rao, Lett. Drug Des. Discov. 6, 21 (2009).
- C. R. Yang, Y. Zang, M. R. Jacob, S. I. Khan, Y. J. Zhang, X. C. Li, Antimicrob. Agents Ch. 50, 1710 (2006).


