ONE-STEP PURIFICATION OF TWO SEMI-SYNTHETIC EPICATECHIN ADDUCTS PREPARED FROM AVOCADO PEELS PROCYANIDINS BY CENTRIFUGAL PARTITION CHROMATOGRAPHY AND EVALUATION OF THEIR ANTI-INFLAMMATORY EFFECTS ON ADENOCARCINOMA GASTRIC CELLS INFECTED WITH Helicobacter pylori
- Centrifugal Partition Chromatography,
- Helicobacter pylori,
- avocado,
- proanthocyanidins,
- IL-8
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
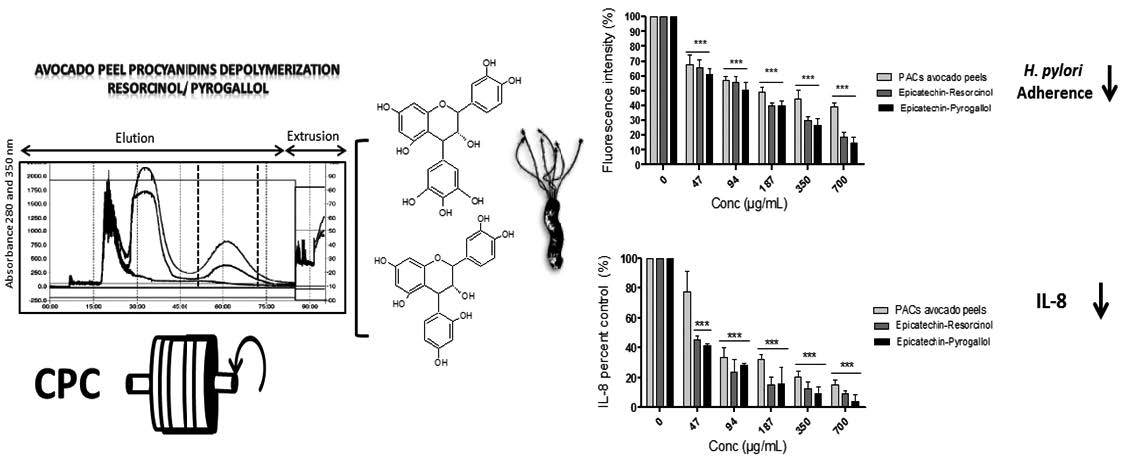
Avocado peels were used to extract proanthocyanidins (PACs) as starting material to prepare semi-synthetic derivatives of epicatechin by nucleophilic attack with pyrogallol and resorcinol. One-step isolation of both semi-synthetic derivatives was successfully performed through centrifugal partition chromatography (CPC), and identified by TLC and LC-MS/MS. The effect upon adherence of Helicobacter pylori ATCC 43504 to AGS cells and the subsequent induction of pro-inflammatory IL-8 release was tested for these two semi-synthetic derivatives. Bacterial adherence and IL-8 synthesis was evaluated using AGS cells con-infected with FITC-labeled H. pylori. Concentration of 700 μg/mL of epicatechin-pyrogallol and epicatechin-resorcinol decreased H. pylori adherence to AGS cells to less than 20% and reduced IL-8 production to less than 10%. In conclusion, semi-synthetic adducts prepared from avocado PACs reduce significantly the adherence and IL-8 production in H. pylori-infected AGS cells.
References
- Fine, AM. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Altern. Med. Rev 2000, 5: 144-51.
- Pastene, E.; Troncoso, M.; Figueroa, G.; Alarcón, J.; Speisky, H. Association between Polymerization Degree of Apple Peel Polyphenols and Inhibition of Helicobacter pylori Urease. J. Agric. Food Chem, 2009, 57: 416–424.
- Pastene, E.; Parada, V.; Avello, M.; Ruiz, A.; García, A. Catechin-based Procyanidins from Peumus boldus Mol. Aqueous Extract Inhibit Helicobacter pylori Urease and Adherence to Adenocarcinoma Gastric Cells. Phytother Res, 2014, 28: 1637-1645.
- Torres, JL.; Lozano, C.; Juliá, L.; Sánchez-Baeza, J.; Anglada, JM.; Centelles, JJ.; Cascante, M. Cysteinyl-flavan-3-ol Conjugates from Grape Procyanidins. Antioxidant and Antiproliferative Properties. Bioorg. Med. Chem, 2002, 10: 2497-2509.
- Mitjans, M.; Martínez, V.; del Campo, J.; Abajo, C.; Lozano, C.; Torres, JT.; Vinardell, P. Novel epicatechin derivatives with antioxidant activity modulate interleukin-1β release in lipopolysaccharide-stimulated human blood. Bioog. & Med. Chem. Lett, 2004, 14: 5031-5034.
- Ugartondo, V.; Mitjans, M.; Lozano, C.; Torres, JL.; Vinardell, MP. Comparative Study of the Cytotoxicity Induced by Antioxidant Epicatechin Conjugates Obtained from Grape. J. Agric. Food Chem, 2006, 54: 6945- 6950.
- Carreras, A.; Mesa, JA.; Cascante, M.; Torres, JL.; Juliá, L. High electron transfer capacity of thio-derivatives of tea catechins measured using a water soluble stable free radical and their effects on colon cancer cells. New J. Chem, 2013, 37: 2043-2050.
- Wroblewski, L.E.; Peek, R.M.; Wilson K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin Microbiol Rev 2010, 23, 713–739.
- Go, M. Natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther, 2002, 16, 3-15.
- Malfertheiner, P.; Megraud, F.; O’Morain, CA.; Gisbert, JP.; Kuipers, EJ.; Axon, AT.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, DY.; Hunt, R.; Moayyedi, P.; Rokkas, T.; Rugge, M.; Selgrad, M.; Suerbaum, S.; Sugano, K.; El-Omar, EM. Management of Helicobacter pylori infection the Maastricht V/ Florence Consensus Report. Gut, 2017, 66: 646-664.
- Ferreccio, C.; Rollán, A.; Harris, PR.; Serrano, C.; Gederlini, A.; Margozzini, P.; Gonzalez, C.; Aguilera, X.; Venegas, A.; Jara, A. Gastric Cancer is related to Early Helicobacter pylori Infection in a High- Prevalence Country. Cancer Epidemiol Biomarkers Prev, 2007, 16: 662- 667.
- Peek, R.M; Crabtree, J.E. 2006. Helicobacter infection and gastric neoplasia. J Patho, 2006, 208, 233–248.
- Sachs, G.; Scott, D. Helicobacter pylori: eradication or preservation. F1000 Med Rep 2012, 4, 7.
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA: CA Cancer J Clin, 2005, 55: 74-108.
- Teixeira Mendes, S.; Attygalle, D.; Wotherspoon, C. Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: a re-evaluation. Gut, 2014, 63, 1526-1527.
- Dacoll, C.; Balter, H.; Varela, L.; Buenavida, G.; González, N.; Silveira, A.; Cohen, H. Evolución de la respuesta al tratamiento de primera línea de la infección por Helicobacter pylori en Uruguay. Acta Gastroenterol Latinoam. 2014, 44: 88-93.
- Martínez, M.; Henao, R.; Lizarazo, R. Resistencia antibiótica de Helicobacter pylori en América Latina y el Caribe. Rev Col Gastroenterol, 2014, 29: 218-227.
- Köhler, N.; Wray, V.; Winterhalter, P. Preparative isolation of procyanidins from grape seed extracts by high-speed counter-current chromatography. J Chromatogr A, 2008, 1177: 114-125.
- Bordiga, M.; Coïsson, JD.; Locatelli, M.; Arlorio, M.; Travaglia, F. Pyrogallol: An Alternative Trapping Agent in Proanthocyanidins Analysis. Food Anal. Methods, 2013, 6: 148-156.
- Glavnik, V.; Simonovska, B.; Vovk, I. Densitometric determination of (+)-catechin and (−)-epicatechin by 4-dimethylaminocinnamaldehyde reagent. J. Chromatogr A, 2009; 1216: 4485-4491.
- National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. Approved standard M7- A5. Informational supplement M100-S10. NCCLS, Wayne, Pa.
- Tolnai, S. A method for viable cell count. Methods Cell Sci, 1975, 1: 37- 38.
- Sarker, SD.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods, 2007, 42: 321-324.
- Beil, W.; Kilian, P. EPs 7630 an extract from Pelargonium sidoides roots inhibits adherence of Helicobacter pylori to gastric cells. Phytomedicine, 2007, 14: 5-8.
- Scott, DR.; Weeks, D.; Hong, C.; Postius, S.; Melchers, K.; Sachs, G. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology, 1998, 114: 58-70.
- Chávez, F.; Aranda, M.; García, A.; Pastene, E. Los polifenoles antioxidantes extraídos del epicarpio de Palta (Persea americana var. Hass) inhiben la ureasa de Helicobacter pylori. BLACPMA 2011; 10: 263- 280.
- Salama, N.; Hartung, M.; Müller, A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microb, 2013, 11: 385-399.
- Marcus, E.; Moshfegh, AP:, Sachs, G.; Scott, DR. The periplasmic a-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J Bacteriol, 2005, 187: 729-738.
- Rohdenwald, P.; Beil, W. In vitro inhibition of Helicobacter pylori growth and adherence to gastric mucosal cells by pycnogenol. Phytother Res, 2008, 22: 685-688.
- Pastene, E.; Speisky, H.; García, A.; Moreno, J.; Troncoso, M.; Figueroa, G. In Vitro and In Vivo Effects of Apple Peel Polyphenols against Helicobacter pylori. J. Agric. Food Chem, 2010, 58: 7172-7179.


