PECTIN; HETERO POLYSACCHARIDE AS A GREEN AND NATURAL CATALYST FOR THE SYNTHESIS OF DIHYDRO-2-OXOPYRROLES AND 3,4,5-TRISUBSTITUTED FURAN-2(5H)-ONES
- Pectin,
- dihydro-2-oxopyrroles,
- 3,
- 4,
- 5-trisubstituted furan-2(5H)-ones
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
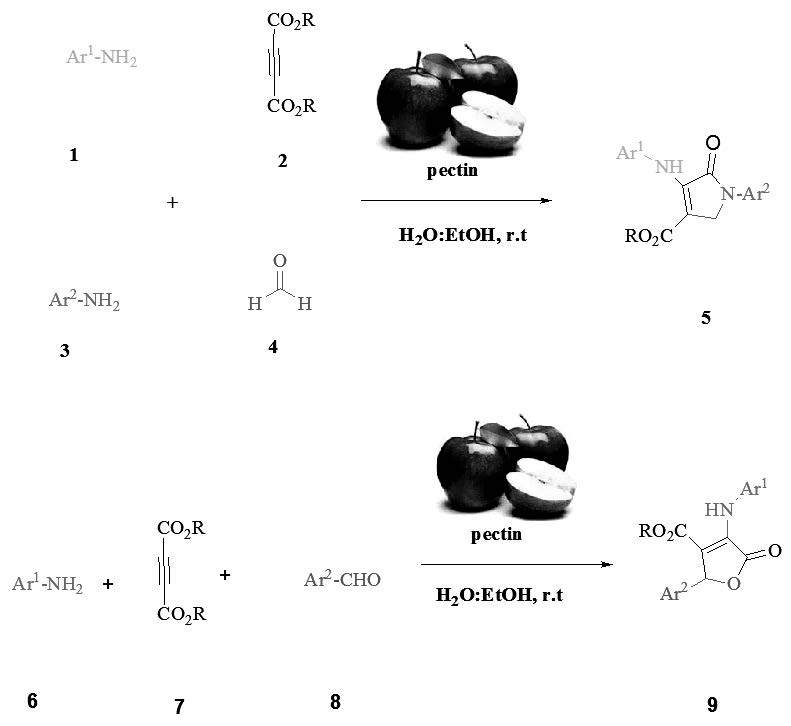
Use of green and biodegradable catalyst in organic synthesis is undeniable. Easy access, inexpensive, natural and green catalysts are valuable. In this study, a green procedure by using pectin as a green and natural catalyst for the one-pot synthesis of dihydro-2-oxopyrroles and 3,4,5-trisubstituted furan-2(5H)-ones at ambient temperature in aqueous media has been developed. This methodology has some advantages such as: use of a hetero polysaccharide as a easy access and biodegradable catalyst, clean work-up and no need to column chromatography.
References
- D.Mohnen, In: Barton D, Nakanishi K, Meth-Cohn O (eds) Comprehensive natural products chemistry, Elsevier,Dordrecht, The Netherlands, 497 (1999).
- M.A. O’Neill , T.Ishii, P. Albersheim, A.G. Darvill, Annu Rev Plant Biol, 55, 109 (2004).
- B.L.Ridley, M.A O’Neill, D.Mohnen, Phytochemistry, 57, 929 (2001).
- M.A. Laurent, P. Boulenguer, Food Hydrocoll, 17,445 (2003).
- G.W.Pilgrim, R.H.Walter, D.G. Oakenfull, In: Walters RH(ed) The chemistry and technology of pectin. Academic Press.Inc., San Diego, 23 (1991).
- W. Pilnik , A.G.J. Voragen, In: Hulme HC (ed) The biochemistry of fruits and their products, Academic Press, London, 1, 53 (1970).
- A. Akansha, A.Deepali, B.Anamika, K.Virendra Kumar, Res. J. Recent. Sci., 364 (2014).
- W.Shi, Y.Duan, Y.Qian, M.Li, L.Yang, W. Hu, Med. Chem. Lett. 20, 3592 (2010).
- X.Luo, M.Shu, Y.Wang, J.Liu, W.Yang, Z.Lin, 3D-QSAR .Molecules. 17, 2015 (2012).
- J. W.Lampe, Y. L.Chou, R. G.Hanna, S. V. D.Meo, P. W. Erhardt, A. A.Hagedorn, Ingebretsen, W. R.; Cantor, E. J. Med. Chem. 36, 1041 ( 1993).
- T.Kawasuji, M.Fuji, T.Yoshinaga, A.Sato, T. Fujiwara, R.Kiyama, Bioorg. Med. Chem. 15, 5487 (2007).
- C.Peifer, R.Selig, K.Kinkel, D.Ott, F.Totzke, C.Schächtele, R.Heidenreich, M. Röcken, D.Schollmeyer, S. Laufer, J. Med.Chem. 51, 3814 (2008).
- T.Bach, H.Brummerhop, Angew. Chem., Int. Ed. 37, 3400 (1998).
- M.Kawase, M. Hirabayashi, S.Saito, K.Yamamoto, Tetrahedron Lett. 40, 2541 (1999).
- D.M.P, Y.M, H.W, G.LS., J. Am. Chem. Soc. 125, 4692 (2003).
- A. J.Clark, C. P. M.C.Dell, , J. M. Donagh, J.Geden, P.Mawdsley, Org. Lett. 5, 2063 (2003).
- J.Chen, P.Q.Huang, Y.Queneau, J. Org. Chem. 74,7457 (2009).
- H.He, H. Y.Yang, R.Bigelis, E. H.Solum, M.Greenstein, G. T. Carter, Tetrahedron Lett. 43, 1633 (2002).
- T.Agatsuma, T. Akama, S.Nara, S.Matsumiya, R.Nakai, H.Ogawa, S.Otaki, S.I.Ikeda, Y.Saitoh, Y. Kanda, Org. Lett. 4, 4387 (2002).
- M. Adib, M. Mahdavi, M. A.Noghani, H. R. Bijanzadeh, Tetrahedron Lett. 48, 8056 (2007).
- M.Aginagalde, T.Bello, C.Masdeu, Y.Vara, A.Arrieta, F. P. Cossío, J. Org. Chem. 75, 7435 (2010).
- F.Palacios, J.Vicario, D. Aparicio, Eur. J. Org. Chem. 2843 (2006).
- J.Zhang, P. G.Blazecka, J. G. Davidson, Org. Lett. 5,553 (2003).
- M.J.Fan, B.Qian, L.B.Zhao, Y.M. Liang, Tetrahedron. 63, 8987 (2007).
- Q.Zhu, H.Jiang, J.Li, S.Liu, C.Xia, M. Zhang, J. Comb. Chem. 11, 685 (2009).
- A. T.Khan, A.Ghosh, M.d. M. Khan, Tetrahedron Lett. 53, 2622 (2012).
- G. J.Hollingworth, G.Perkins, J. B.Sweeney, J. Chem. Soc., Perkin Trans. 1, 913 (1996).
- R.Mabon, A. M. E. Richecoeur, J. B. Sweeney, J. Org. Chem. 64, 328 (1999).
- R. Mabon, A. M. E.Richecoeur, J. B. Sweeney, Tetrahedron. 58, 9117 (2002).
- M.Bassetti, A. D’Annibale, A.Fanfoni, F. Minissi, Org. Lett. 7, 1805 (2005).
- A.Yazdani Elah Abadi, M.T. Maghsoodlou, R.Heydari, R. Mohebat, Res. Chem.Int. doi: 10.1007/s11164-015-2083-5(2015).
- M.Kngani, N.Hazeri, M.T. Maghsoodlou, J.Saud.Chem.Soc. doi:10.1016/j. jscs.2015.03.002 (2015).
- A.Moradi, R.Heydari, M.T. Maghsoodlou, Res.Chem.Int. doi: 10.1007/ s11164-014-1818-z (2014).
- N.Hazeri, S. S.Sajadikhah, M. T.Maghsoodlou, S.Mohamadian-Souri, M.Norouzi, M. Moein, Chin.Chem.Soc. 61,217 (2013).
- S. S.Sajadikhah, N.Hazeri, M. T. Maghsoodlou, S. M. Habibi-Khorassani, Chin.Chem.Soc. 60, 1003 (2013).
- N.Hazeri, M.T. Maghsoodlou, S. M.Habibi-Khorassani, J. Aboonajmi, S. S. Sajadikhah, Chin.Chem.Soc. 60, 355 (2013).
- R.Doostmohammadi, M.T.Maghsoodlou, N.Hazeri, S.M. Habibi- Khorassani, Chin.Chem.lett, 24, 901 (2013).
- P. T. Anastas, J. C. Warner, Oxford university press (2000).
- Q.Zhu, H.Jiang, J. Li, ,S.Liu, C.Xia, , M. Zhang, J.Com. Chem., 11, 685 (2009).
- A. T.Khan, A.Ghosh, M. M.Khan, Tetrahedron Lett. 53, 2622 (2012).
- S. S.Sajadikhah, N.Hazeri, M. T.Maghsoodlou, S. M. Habibi-Khorassani, A.Beigbabaei, A. C.Willis, J.Iran. Chem. Soc. 10, 863 (2013).
- S. N.Murthy, B.Madhav, A. V.Kumar, K. R. Rao, Y. V. D. Nageswar, Tetrahedron, 65, 5251 (2009).
- L.Nagarapu, U. N.Kumar, P. Upendra, R. Bantu, Syn. Commu. 42, 2139 (2012).


