SALICYLIC ACID AS AN EFFICIENT CATALYST FOR THE DIASTEREOSELECTIVE SYNTHESIS OF DISPIROHYDROQUINOLINES VIA A ONE-POT DOMINO EIGHT-COMPONENT REACTION
- Aromatic aldehydes,
- Aromatic amines,
- Meldrum’s acid,
- Dispiro compounds
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
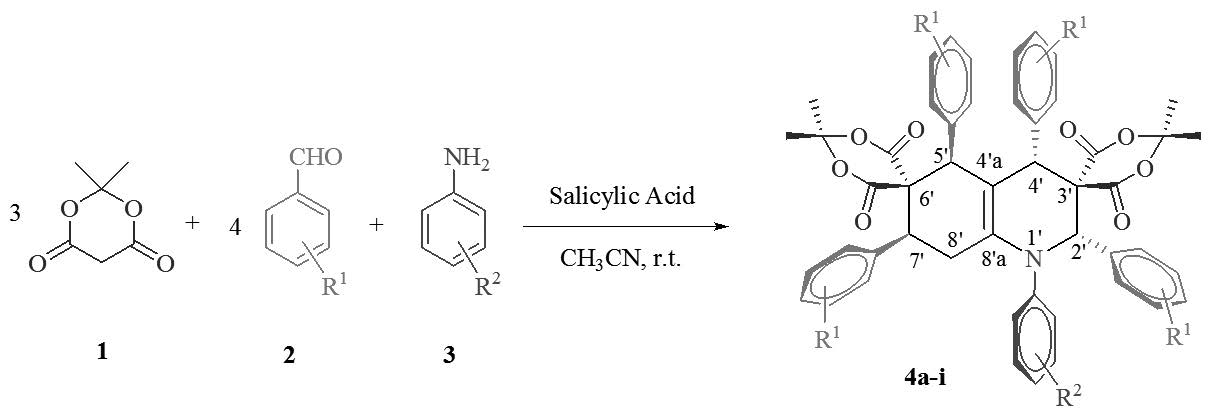
Salicylic acid was used as an efficient catalyst for the diastereoselective synthesis of dispirohydroquinoline-bis (Meldrim’s acid) derivatives via a one-pot domino eight-component reaction of arylamines, aromatic aldehydes and Meldrum’s acid in CH3CN at room temperature. The remarkable advantages offered by this method are inexpensive catalyst, good yields, simple and easy work-up procedure. The characterization of products was done by IR, mass, 1H NMR, 13C NMR spectroscopy, and elemental analyses. The stereoselectivity of compounds was confirmed with crystallography and NMR spectroscopy.
References
- Dömling, Chem. Rev. 106, 17 (2006).
- R.V.A. Orru, M. de Greef, Synthesis 10, 1471 (2003).
- Hulme, V. Gore, Curr. Med. Chem. 10, 51 (2003).
- Montagne, J.J Shiers, M. Shipman, Tetrahedron Lett. 47, 9207 (2006).
- R.W. Carling, P.D. Leeson, A.M. Moseley, R. Baker, A.C. Forster, S. Grimwood, J.A. Kemp, G.R. Marshall, J. Med. Chem. 35, 1942 (1992).
- S. X. Cai, Z-L. Zhou, J-C. Huang, E.R. Whittermore, Z.O. Egbuwoku, J.E. Hawkinson, R.M. Woodward, J.F. Keana, J. Med. Chem. 39, 3248 (1996).
- I. Jacquemond-Collet, F. Benoit-Vical, A. Valentin, E. Stanislas, M. Mallie, I. Fouraste, Planta Med. 68, 68 (2002).
- O.B. Wallace, K.S. Lauwers, S.A. Jones, J.A. Dodge, Bioorg. Med. Chem. Lett. 13, 1907 (2003).
- Jacobs, M. Frotscher, G. Dannhardt, R.W. Hartmann, J. Med. Chem. 43, 1841 (2000).
- G. Dorey, B. Lockhart, P. Lestage, P. Casara, Bioorg. Med. Chem. Lett. 10, 935 (2000).
- T.T. Nishiyama, Y.Y. Hashiguchi, S.T. Sakata, T.T. Sakaguchi, Polym. Degrad. Stab.79, 225 (2003).
- W. Calhoun, R.P. Carlson, R. Crossley, L.J. Datko, S. Dietrich, K. Heatherington, L.A. Marshall, P.J. Meade, A. Opalko, R.G. Shepherd, J. Med. Chem. 38, 1473 (1995).
- Schmittel, M. Strittmatter, K. Vollmann, S. Kiau, Tetrahedron Lett. 37, 999 (1996).
- H.A. Etman, M.A. Sofan, M.A. Metwally, Boll. Chim. Farm. 134, 249 (1995).
- Hazeri, M. T. Maghsoodlou, S. M. Habibi-Khorassani, G. Marandi, K. Khandan-Barani, M. Ziyaadini, A. Aminkhani, ARKIVOC i, 173 (2007).
- M.T. Maghsoodlou, S.M. Habibi-Khorassani, A. Moradi, N. Hazeri, A. Davodi, S. S. Sajadikhah, Tetrahedron 67, 8492 (2011).
- M.T. Maghsoodlou, S.M. Habibi-Khorassani, R. Heydari, F. Rostami- Charati, N. Hazeri, M. Lashkari, M. Rostamizadeh, G. Marandi, A. Sobolev, M. Makha, Tetrahedron Lett. 50, 4439 (2009).
- N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, M. Ziyaadini, G. Marandi, K. Khandan-Barani, H.R. Bijanzadeh, ARKIVOC xiii, 34 (2007).
- Salahi, M. T. Maghsoodlou, N. Hazeri, M. Lashkari, N. Akbarzadeh Torbati, M.A. Kazemian, S. García-Granda, L. Torre-Fernández J. Saudi Chem. Soc. 20, 349 (2016).
- Salahi, M. T. Maghsoodlou, N. Hazeri, M. Lashkari, S. Garcia-Granda, L. Torre-Fernandez Chin. J. Catal. 36, 1023 (2015).
- M. Lashkari, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, N. Akbarzadeh-Torbati, S. García-Granda, L. Torre-Fernández, J. Heterocyclic Chem., 52, 873 (2015).
- S. Salahi, N. Hazeri, M.T. Maghsoodlou, S. García-Granda, L. Torre- Fernández, J. Chem. Res. 38, 383 (2014).
- N. Hazeri, M. Lashkari, S. García-Granda, L. Torre-Fernández, Aust. J. Chem. 67, 1656 (2014).
- D.B. Ramachary, N.S. Chowdari, C.F. Barbas III, Angew. Chem. Int. Ed. 42, 4233 (2003).
- D.B. Ramachary, K. Anebousely, N.S. Chowdari, C.F. Barbas III, J. Org. Chem. 69, 5838 (2004).
- D.B. Ramachary, N.S. Chowdari, C.F. Barbas III, Synlett 12, 1910 (2003).
- B. Jiang, W.-J. Hao, J.-P. Zhang, S.-J. Tu, F. Shi, Org. Biomol. Chem. 7, 2195 (2009).
- E.S. Shults, E.A. Semenov, A.A. Johnson, S.P. Bondarenko, I.Y. Bagryanskaya, Y.V. Gatilov, G.A. Tolstikov, Y. Pommier, Bioorg. Med. Chem. Lett. 17, 1362 (2007).
- J. Shi, Y. Liu, M. Wang, L. Lin, X. Liu, X. Feng, Tetrahedron 67, 1781 (2011).
- Pizzirani, M. Roberti, M. Recanatini, Tetrahedron Lett. 48, 7120 (2007).
- W.-J. Hao, B. Bo Jiang, S.-J. Tu, S.-S. Wu, Z.-G. Han, X.-D. Cao, X.-H. Zhang, S. Yan, F. Shi, J. Comb. Chem. 11, 310 (2009).
- D. Pizzirani, M. Roberti, S. Grimaudo, A. Di Cristina, R.M. Pipitone, M. Tolomeo, M. Recanatini, J. Med. Chem. 52, 6936 (2009).
- P. Wang, L. Song, H. Yi, M. Zhang, S. Zhu, H. Deng, M. Shao, Tetrahedron Lett. 51, 3975 (2010).
- S.-J. Tu, X. Zhu, J. Zhang, J. Xu, Y. Zhang, Q. Wang, R. Jia, B. Jiang, J. Zhang, C. Yao, Bioorg. Med. Chem. Lett. 16, 2925 (2006).
- X.-S. Wang, M.-M. Zhang, H. Jiang, C.-S. Yao, S.-J. Tu, Tetrahedron. 21, 4439 (2007).
- J. Sun, E.-Y. Xia, Q. Wu, C.-G. Yan, ACS Comb. Sci. 13, 421 (2011).
- Agilent (2013). CrysAlis PRO. Agilent Technologies UK Ltd, Yarnton, England.
- M.C. Burla, R. Caliandro, M. Camalli, B. Carrozzini, G.L. Cascarano, C. Giacovazzo, M. Mallamo, A. Mazzone, G. Polidori, R. Spagna, J. Appl. Cryst. 45, 357 ( 2012).
- G.M. Sheldrick, Acta Cryst. 64, 112 (2008).
- L.J. Farrugia, J. Appl. Cryst. 30, 565 (1997).
- L.J. Farrugia, J. Appl. Cryst. 32, 837 (1999).


