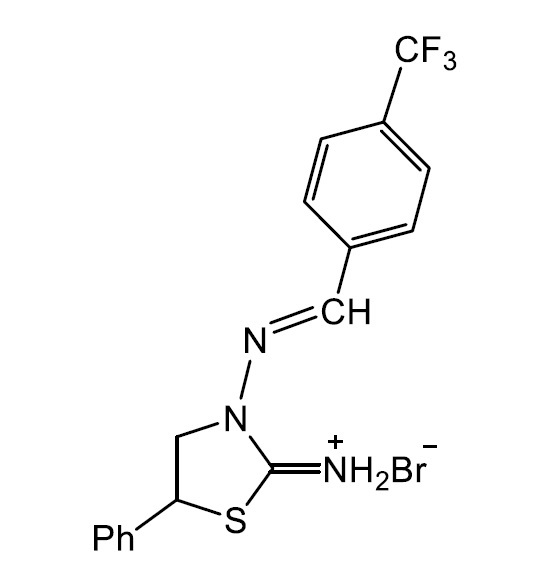
CRYSTAL STRUCTURE AND HIRSHFELD SURFACE ANALYSIS OF (E)-5-PHENYL-3-((4- (TRIFLUOROMETHYL)BENZYLIDENE)AMINO)THIAZOLIDIN-2-IMINIUM BROMIDE
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
In the cation of the title salt, the central thiazolidine ring adopts an envelope conformation. In the crystal N-H ··· Br hydrogen bonds link the components into a bi-dimensional network with the cations and anions stacked parallel to plane (101). The molecular structure shows several positional disorders over -CF3 and thiazolidine fragments and these were modeled. The weak intermolecular interactions in the crystal structure are mainly constituted by H ··· F, H ··· π and H ··· Br. Hirshfeld surface analysis were used to verify the contributions of the different intermolecular interactions.
References
- G. Mahmoudi, F. A. Afkhami, A. Castiñeiras, I. García-Santos, A. Gurbanov, F. I. Zubkov, M. P. Mitoraj, M. Kukułka, F. Sagan, D. W. Szczepanik, I. A. Konyaeva and D. A. Safin, Inorg. Chem., 2018, 57, 4395– 4408.
- G. Mahmoudi, A. Bauzá, A. V. Gurbanov, F. I. Zubkov, W. Maniukiewicz, A. Rodríguez-Diéguez, E. López-Torres and A. Frontera, CrystEngComm, 2016, 18, 9056–9066.
- K. T. Makhmudov, R. A. Alieva, S. R. Gadzhieva and F. M. Chyragov, J. Anal. Chem., 2008, 63, 435–438.
- A. M. Maharramov, R. A. Alieva, K. T. Mahmudov, A. V Kurbanov and R. K. Askerov, Russ. J. Coord. Chem., 2009, 35, 704–709.
- D. Y. Vandyshev, K. S. Shikhaliev, A. Y. Potapov, M. Y. Krysin, F. I. Zubkov and L. V. Sapronova, Beilstein J. Org. Chem., 2017, 13, 2561–2568.
- D. K. Nasirova, A. V. Malkova, K. B. Polyanskii, K. Y. Yankina, P. N. A. Amoyaw, I. A. Kolesnik, A. V. Kletskov, I. A. Godovikov, E. V. Nikitina and F. I. Zubkov, Tetrahedron Lett., 2017, 58, 4384–4387.
- E. A. Kvyatkovskaya, V. P. Zaytsev, F. I. Zubkov, P. V. Dorovatovskii, Y. V. Zubavichus and V. N. Khrustalev, Acta Crystallogr. Sect. E Crystallogr. Commun., 2017, 73, 515–519.
- A. A. Shetnev and F. I. Zubkov, Chem. Heterocycl. Compd., 2017, 53, 495– 497.
- A. V. Gurbanov, A. M. Maharramov, F. I. Zubkov, A. M. Saifutdinov and F. I. Guseinov, Aust. J. Chem., 2018, 71, 190–194.
- K. T. Mahmudov, M. N. Kopylovich, A. Sabbatini, M. G. B. Drew, M. D. R. S. Martins, C. Pettinari and A. J. L. Pombeiro, Inorg. Chem., 2014, 53, 9946–9958.
- R. Jlassi, A. P. C. Ribeiro, M. F. C. Guedes Da Silva, K. T. Mahmudov, M. N. Kopylovich, T. B. Anisimova, H. Naïli, G. A. O. Tiago and A. J. L. Pombeiro, Eur. J. Inorg. Chem., 2014, 2014, 4541–4550.
- A. V. Gurbanov, G. Mahmoudi, M. F. C. Guedes da Silva, F. I. Zubkov, K. T. Mahmudov and A. J. L. Pombeiro, Inorganica Chim. Acta, 2018, 471, 130–136.
- N. Q. Shikhaliyev, N. E. Ahmadova, A. V. Gurbanov, A. M. Maharramov, G. Z. Mammadova, V. G. Nenajdenko, F. I. Zubkov, K. T. Mahmudov and A. J. L. Pombeiro, Dye. Pigment., 2018, 150, 377–381.
- A. V. Gurbanov, K. T. Mahmudov, M. N. Kopylovich, F. M. Guedes da Silva, M. Sutradhar, F. I. Guseinov, F. I. Zubkov, A. M. Maharramov and A. J. L. Pombeiro, Dye. Pigment., 2017, 138, 107–111.
- K. T. Mahmudov and A. J. L. Pombeiro, Chem. - A Eur. J., 2016, 22, 16356– 16398.
- K. T. Mahmudov, M. N. Kopylovich, M. F. C. Guedes Da Silva and A. J. L. Pombeiro, Dalt. Trans., 2017, 46, 10121–10138.
- J. D. Van der Waals, University of Leiden, Leiden., 1873.
- Bruker AXS INC., APEX3, SAINT and SADABS, 2016, Bruker AXS Inc., Madison, Wisconsin, USA.
- G. M. Sheldrick, Acta Crystallogr. Sect. C Struct. Chem., 2015, 71, 3–8.
- O. V Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341.
- C. D. Centre and L. Road, 1986, 13, 4343–4348.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer and A. G. Orpen, 1987, 1–19.
- A.N.Khalilov, Z.Atioğlu, M.Akkurt, G.S.Duruskari, F.A.A.Toze and A.T.Huseynova. Acta Cryst., 2019, E75, 662-666.
- M.Akkurt, G.Sh.Duruskari, F.A.A.Toze, A.N.Khalilov and A.T.Huseynova. Acta Cryst., 2018, E74, 1168-1172.
- M.Akkurt, A.M.Maharramov, G.S.Duruskari, F.A.A.Toze and A.N.Khalilov. Acta Cryst., 2018, E74, 1290-1294.
- J. J. McKinnon, D. Jayatilaka and M. A. Spackman, Chem. Commun., 2007, 3814–3816.
- M. A. Wolff, S.K., Grimwood, D.J., McKinnon, J.J., Turner, M.J., Jayatilaka, D. and Spackman, 2012, Crystal Explorer 17.5. University of Western Austr.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392.


