BENCH-SCALE EXTRACTION OF STILBENOIDS AND OTHER PHENOLICS FROM STORED GRAPE CANES (VITIS VINIFERA): OPTIMIZATION PROCESS, CHEMICAL CHARACTERIZATION, AND POTENTIAL PROTECTION AGAINST OXIDATIVE DAMAGE
- Stilbenoids,
- Procyanidins,
- Grape canes,
- Bench-scale extraction,
- Antioxidant capacity
Copyright (c) 2019 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
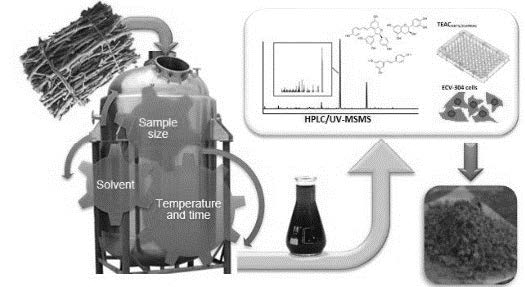
Dietary supplements have become the key to complement deficiencies in the occidental diet and therefore to reduce the incidence of oxidative stress related diseases. A bench-scale extraction procedure was studied to obtain a valuable product rich in phenolic compounds and antioxidant capacity from Pinot Noir grape cane enhanced by storage. Extraction solvent, cane-size, solid:liquid ratio, temperature, and extraction time, were systematically evaluated in order to obtain a natural functional product. Complete chemical characterization of a Pinot Noir grape cane extract produced under bench scale process is presented for the first time. Phenolic profiles of the extracts were characterized by HPLC-PDA-MS/MS and minerals by ICP-OES. Proteins, carbohydrates and lignins were also evaluated. The main phenolic compounds in the final product were stilbenoids, flavan-3-ols, procyanidins, and flavonols, with 6.53%, 4.84%, 2.11%, and 0.25%, respectively on a dry matter basis. Other chemical constituents were carbohydrates (27%), minerals (1%) and lignins (38.7%). The antioxidant capacity of the product was demonstrated using chemical assays (TEACABTS/CUPRAC and ORAC-FL) and endothelial cells model. The extract produced under the described bench scale process using grape cane enhanced by storage have a chemical composition and protecting capacities to be used in functional foods industry.
References
- Boue SM, ClevelandTE, Carter-Wientjes C, Shih BY, BhatnagarD, McLachlanJM, BurowME. Phytoalexin-Enriched Functional Foods. J Agric Food Chem 57: 2614–2622 (2009).
- Diaz-Gerevini GT, Repossi G, Dain A, Tarres MC, Das UN, Eynard AR. Beneficial action of resveratrol: How and why?. Nutrition 32:174–8 (2016).
- Bagchi, D.; Sen, C.K.; Ray, S.D.; Das, D. Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res 523–524: 87-97(2003).
- Lizarraga, D.; Lozano, C.; Briedé, J. J.; van Delft, J. H. The importance of polymerization and galloylation for the antiproliferative properties of procyanidin-rich natural extracts. FEBS J 274: 4802–4811 (2007).
- Llopiz, N.; Puiggro, F.;Céspedes, E.; Arola, L. Antigenotoxic effect of grape seed procyanidin extract in FAO cells submitted to oxidative stress. J Agric Food Chem 52: 1083–1087 (2004).
- Gabaston J, Cantos-Villar E,BiaisB,Waffo-TeguoP,Renouf E,Corio-Costet M, Richard T,Mérillon J. Stilbenes from Vitisvinifera L. Waste: A Sustainable Tool for Controlling PlasmoparaViticola. J Agric Food Chem 65: 2711–2718 (2017).
- Flamini, R.; Rosso, M. De.; Marchi, F. De. An innovative approach to grape metabolomics: stilbene profiling by suspect screening analysis, Int J Mol Sci 14:1243–1253(2012).
- Vergara C, Von BaerD, Mardones C,Wilkens A,Wernekinck K,Damm A, Macke S,Gorena T,Winterhalter P. Stilbene levels in grape cane of different cultivars in southern Chile: Determination by HPLC-DADMS/MS method. J Agric Food Chem 60: 929–933 (2012).
- Gorena, T.; Sáez, V.; Mardones, C.; Vergara, C.; Winterhalter, P.; von Baer, D. (2014). Influence of post-pruning storage on stilbenoid levels in Vitis vinifera L. canes. Food Chem 155: 256–263 (2014).
- Karacabey, E.; Mazza, G. Optimization of solid-liquid extraction of resveratrol and other phenolic compounds from milled grape canes (Vitisvinifera). J Agric Food Chem 56: 6318–6325 (2008).
- Karacabey, E.; Bayindirli, L.; Artik, N.; Mazza, G. Modeling solid-liquid extraction kinetics of trans-resveratrol and trans-ε-viniferin from grape cane. J Food ProcessEng 36: 103–112 (2013).
- Piñeiro, Z.; Guerrero, R. F.; Fernández-Marin, M. I.; Cantos-Villar, E.; Palma, M. Ultrasound-assisted extraction of stilbenoids from grape stems. J Agric Food Chem 61: 12549–12556 (2013).
- Bart, H. J. &Pilz, S. Industrial Scale Natural Products Extraction. (Wiley, 2011).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 analytical procedure - Determination of structural carbohydrates and lignin in Biomass. https://www.nrel.gov/docs/gen/fy13/42618.pdf [2 October 2015].
- Ku, C. S.; Mun, S. P. Characterization of proanthocyanidin in hot wáter extract isolated from Pinus radiata bark. Wood Sci Technol 41: 235–247 (2007).
- Çetin, E. S.; Altinöz, D.;Tarçan, E.; GöktürkBaydar, N. Chemical composition of grape canes. Ind Crops Prod 34: 994–998 (2011).
- Ribeiro, J.P.N.; Magalhães, L.M.; Reis, S.; Lima, J.L.F.C.; Segundo, M. A. High-throughput total cupric ion reducing antioxidant capacity of biological samples determined using flow injection analysis and microplate-based methods. Anal Sci 27: 483–488 (2011).
- Karacabey, E. & Mazza, G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem 119: 343–348 (2010).
- Wolfe, K.L.; Rui, H.L. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55: 8896–8907(2007).
- Brewer, L. R.; Kubola, J.; Siriamornpun, S.; Herald, T. J.; Shi, Y. C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem 152: 483–490 (2014).
- Hemery, Y. M.; Anson, N. M.; Havenaar, R.; Haenen, G. R. M. M.; Noort, M. W. J.; Rouau, X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res Int 43: 1429–1438 (2010).
- Gil, M.; González, A.; Gil, A.Evaluation of milling energy requirements of biomass residues in a semi-industrial pilot plant for co-firing. http://www.bioscale.es/wpcontent/uploads/downloads/2014/04/Evaluation-of-milling-energyrequirements-of-biomass-residues-for-cofiring.pdf [11 [16March 2016].
- Gabaston J, Cantos –Villar E, Biais B, Waffo-Teguo P, Renouf E, Corio-Costet M, Richard T, Mérillon J. Stilbenes from Vitisvinifera L. Waste: A Sustainable Tool for Controlling Plasmoparaviticola. J. Agric Food Chem 65: 2711-2718 (2017).
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J Agric Food Chem 62: 10861–10872 (2014).
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res Int 65: 462–468(2014).
- Skroza, D.; Generalić, I.; Svilović, S.; Šimat, V.;Katalinić, V. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J Food Comp. Anal 38:13–18 (2015).
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the south Andes region of South America. J.Agric Food Chem 260: 8851–8859 (2012).


